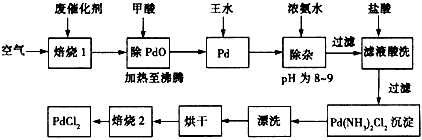
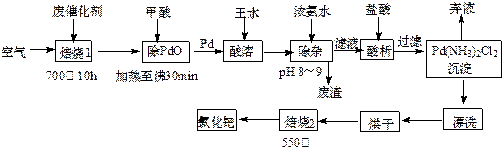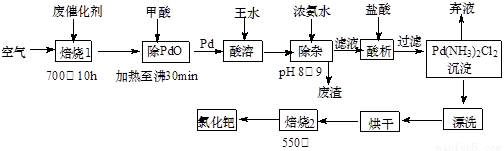��Ŀ����
�л��ϳ��г��õ���/����̿����������ʹ�ã��ᱻ�����л��������������Ⱦ��ʧȥ���ԣ���Ϊ�ϴ�����һ���ɷϴ�����ȡPdCl2�Ĺ����������£�
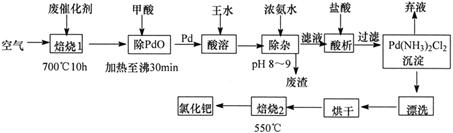
��1��������1��ͨ�������Ŀ���� ��
��2�������ڷ�Ӧ�б�����Ϊ������̼��д��������PdO��Ӧ�Ļ�ѧ����ʽ�� ��
��3������Ũ��ˮ�Ĺ����У���Ҫ������Һ��pHΪ8��9��ʵ�����м����ҺpH�ļ����� ��
��4��д��������2��������Ӧ�Ļ�ѧ����ʽ�� ��
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ�A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%����A�Ļ�ѧʽΪ ��

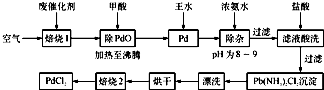
��1��������1��ͨ�������Ŀ����
��2�������ڷ�Ӧ�б�����Ϊ������̼��д��������PdO��Ӧ�Ļ�ѧ����ʽ��
��3������Ũ��ˮ�Ĺ����У���Ҫ������Һ��pHΪ8��9��ʵ�����м����ҺpH�ļ�����
��4��д��������2��������Ӧ�Ļ�ѧ����ʽ��
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ�A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%����A�Ļ�ѧʽΪ
��������1��ʹ�л���ȿ�ȼ����ȼ�գ�
��2�����������Ϣȷ����Ӧ���������Ӷ�д����Ӧ����ʽ��
��3��ʵ��������pH��ֽ�����Һ��pH��
��4�����������Ϣ���ԭ���غ�ȷ����Ӧ����ʽ��
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ���B��һ��������A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%��ʣ�������Ԫ�أ����ݸ�Ԫ�ص���������ȷ����ԭ�Ӹ����ȣ��Ӷ�ȷ���仯ѧʽ��
��2�����������Ϣȷ����Ӧ���������Ӷ�д����Ӧ����ʽ��
��3��ʵ��������pH��ֽ�����Һ��pH��
��4�����������Ϣ���ԭ���غ�ȷ����Ӧ����ʽ��
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ���B��һ��������A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%��ʣ�������Ԫ�أ����ݸ�Ԫ�ص���������ȷ����ԭ�Ӹ����ȣ��Ӷ�ȷ���仯ѧʽ��
����⣺��1����/����̿����������ʹ�ã��ᱻ�����л��������������Ⱦ��ʧȥ���ԣ���ô����к����л��Ϊ��ʹ�л���ȿ�ȼ����ȼ�գ�Ҫ���������������
�ʴ�Ϊ��ʹ�л�������ȿ�ȼ��ͨ��ȼ�ն�������
��2�����������Ϣ֪�����ᱻ�������ɶ�����̼������������ﱻ��ԭ���ɽ������ʣ�ͬʱ�÷�Ӧ�л�����ˮ����Ӧ����ʽΪ��HCOOH+PdO�TPd+CO2��+H2O��
�ʴ�Ϊ��HCOOH+PdO�TPd+CO2��+H2O��
��3��ʵ��������pH��ֽ�����Һ��pH������������ǣ��ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ�Ȼ��pH��ֽ�����ɫ���Աȣ��ʴ�Ϊ���ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ�Ȼ��pH��ֽ�����ɫ���Աȣ�
��4�����������Ϣ֪����Ӧ����Pd��NH3��2Cl2���������ǰ�����PdCl2����Ӧ�����Ǽ��ȣ��÷�Ӧ����ʽΪ��
Pd��NH3��2Cl2
2NH3��+PdCl2��
�ʴ�Ϊ��Pd��NH3��2Cl2
2NH3��+PdCl2��
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ���B��һ��������A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%������Ԫ���غ�֪ʣ�������Ԫ�أ�Pdԭ�ӡ���ԭ�Ӻ���ԭ�Ӹ�����=
��
��
=1��2��4������A�Ļ�ѧʽΪ��H2PdCl4��
�ʴ�Ϊ��H2PdCl4��
�ʴ�Ϊ��ʹ�л�������ȿ�ȼ��ͨ��ȼ�ն�������
��2�����������Ϣ֪�����ᱻ�������ɶ�����̼������������ﱻ��ԭ���ɽ������ʣ�ͬʱ�÷�Ӧ�л�����ˮ����Ӧ����ʽΪ��HCOOH+PdO�TPd+CO2��+H2O��
�ʴ�Ϊ��HCOOH+PdO�TPd+CO2��+H2O��
��3��ʵ��������pH��ֽ�����Һ��pH������������ǣ��ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ�Ȼ��pH��ֽ�����ɫ���Աȣ��ʴ�Ϊ���ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ�Ȼ��pH��ֽ�����ɫ���Աȣ�
��4�����������Ϣ֪����Ӧ����Pd��NH3��2Cl2���������ǰ�����PdCl2����Ӧ�����Ǽ��ȣ��÷�Ӧ����ʽΪ��
Pd��NH3��2Cl2
| ||
�ʴ�Ϊ��Pd��NH3��2Cl2
| ||
��5��Pd�м�����ˮ�ķ�Ӧ���Ա�ʾΪPd+HCl+HNO3-��A+B��+H2O��δ��ƽ��������BΪ��ɫ�ж����壬�������ڿ����в����ȶ����ڣ���B��һ��������A�к�������Ԫ�أ�����PdԪ�ص���������Ϊ42.4%��HԪ�ص���������Ϊ0.8%������Ԫ���غ�֪ʣ�������Ԫ�أ�Pdԭ�ӡ���ԭ�Ӻ���ԭ�Ӹ�����=
| 42.4% |
| 106.4 |
| 0.8% |
| 1 |
| 1-42.4%-0.8% |
| 35.5 |
�ʴ�Ϊ��H2PdCl4��
���������⿼�����Ʊ���������ƣ��漰��ѧʽ��ȷ����������ԭ��Ӧ��pH�IJⶨ��֪ʶ�㣬�����ԭ���غ㼰ԭ�Ӹ�����ȷ����ѧʽ����ȷ��ҺpHֵ�IJⶨ�������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ