��Ŀ����
��֪��Ӧ��
 ��
��
 ��������A~I��ת����ϵ����ͼ��
��������A~I��ת����ϵ����ͼ��

 ��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺
��B�ķ���ʽΪC8H8O���䱽���ϵ�һԪȡ����ֻ�����֣�GΪ�߷��ӻ������ش��������⣺ (1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� ��
(1)д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ�� ����Ӧ�� �� (2)д���������ʵĽṹ��ʽ��F ��I ��A ��
(2)д���������ʵĽṹ��ʽ��F ��I ��A �� (3)д�����з�Ӧ�Ļ�ѧ����ʽ��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ�� ��B��C�� ��
��B��C�� �� ��C+D��H�� ��
��C+D��H�� �� ��F��G�� ��
��F��G�� �� (4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��
(4)C��ͬ�ط��칹������������ķ����廯���ﹲ�� �֣���д������һ��ͬ���칹��Ľṹ��ʽ�� ��
(1)���ۣ�������(2) HOCH2�� ��COOH��HOOC��
��COOH��HOOC�� ��COOH��
��COOH�� ��CH3��
��CH3�� (3)��H3C��
(3)��H3C�� ��CHO + 2Cu(OH)2
��CHO + 2Cu(OH)2 H3C��
H3C�� ��COOH + Cu2O��+ 2H2O��
��COOH + Cu2O��+ 2H2O��
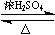 ��H3C��
��H3C�� ��COOH+HOCH2��
��COOH+HOCH2�� ��CH3 H3C��
��CH3 H3C�� ��COOCH2��
��COOCH2�� ��CH3+H2O
��CH3+H2O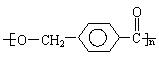

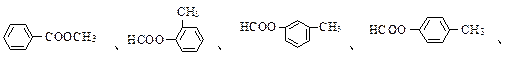
 ��nHOCH2��
��nHOCH2�� ��COOH + nH2O
��COOH + nH2O (4)6��
(4)6��

 ����дһ�֡�
����дһ�֡�
����



 ��
��





