��Ŀ����
 д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ
д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽC+2H2SO4��Ũ��
CO2��+2SO2��+2H2O
| ||
C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O
��
| ||
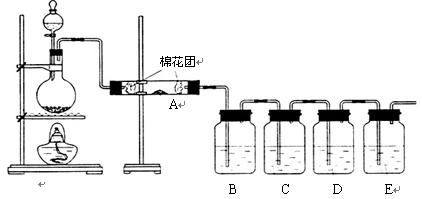
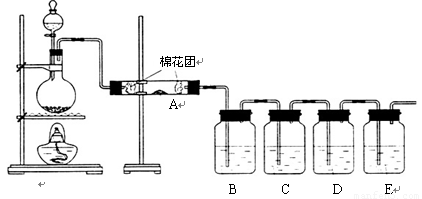
Ϊ����֤�����еĶ�����̼���壬�ס�����ͬѧ�ֱ��������ʵ�飨��֪Br2+2H2O+SO2�TH2SO4+2HBr����
��ش���������
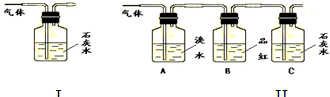
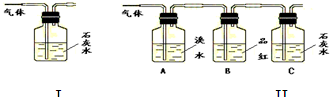
��1�����ݢ�װ����ʯ��ˮ����ǵ������ܷ�˵��������һ���ж�����̼���壿
����
����
����������ΪSO2��ʯ��ˮ��Ӧ����CaCO3Ҳ������ˮ
��ΪSO2��ʯ��ˮ��Ӧ����CaCO3Ҳ������ˮ
����2����װ����A��������
��ȥSO2
��ȥSO2
��B������������SO2�Ƿ��ѳ���
����SO2�Ƿ��ѳ���
����3������B��Ʒ��
B��Ʒ�첻��ɫ
B��Ʒ�첻��ɫ
��C�в���C�в������ǣ����ɫ������
C�в������ǣ����ɫ������
��˵��������һ���ж�����̼���壮����������ľ̿����Ũ���ᷢ����Ӧ��д��ѧ����ʽ��
����S02��CO2��������еĶ�����̼��Ӧ�ų���������ĸ��ţ����������ɰѶ�������������ͬʱ��Ʒ����Һ�����Ƿ����������ʯ��ˮ����Dz���˵����CO2��
����S02��CO2��������еĶ�����̼��Ӧ�ų���������ĸ��ţ����������ɰѶ�������������ͬʱ��Ʒ����Һ�����Ƿ����������ʯ��ˮ����Dz���˵����CO2��
����⣺����ľ̿����Ũ���ᷢ����Ӧ��д��ѧ����ʽ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
��1����S02��C02����ʹʯ��ˮ����ǣ����Ҫ������C02��Ӧ�ȳ���S02������ʯ��ˮ����Dz���˵����C02��
�ʴ�Ϊ������ ��ΪSO2��ʯ��ˮ��Ӧ����CaSO3Ҳ������ˮ��
��2����S02��C02����ʹʯ��ˮ����ǣ����Ҫ������C02��Ӧ�ȳ���S02�����ݶ���������л�ԭ�ԣ�
������ˮ�Ѷ����������գ���Ӧ����ʽΪ��Br2+SO2+2H2O=2HBr+2H2SO4���ɸ��ݶ���������ʹƷ����ɫ��������������Ƿ������
�ʴ�Ϊ����ȥSO2 ����SO2�Ƿ��ѳ�����
��3��Ҫ���������̼��Ӧ�ų����������ݶ�����̼�����ʯ��ˮ����������������̼��
��Ӧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��
�ʴ�Ϊ��B��Ʒ�첻��ɫ C�в������ǣ����ɫ������
| ||
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
��1����S02��C02����ʹʯ��ˮ����ǣ����Ҫ������C02��Ӧ�ȳ���S02������ʯ��ˮ����Dz���˵����C02��
�ʴ�Ϊ������ ��ΪSO2��ʯ��ˮ��Ӧ����CaSO3Ҳ������ˮ��
��2����S02��C02����ʹʯ��ˮ����ǣ����Ҫ������C02��Ӧ�ȳ���S02�����ݶ���������л�ԭ�ԣ�
������ˮ�Ѷ����������գ���Ӧ����ʽΪ��Br2+SO2+2H2O=2HBr+2H2SO4���ɸ��ݶ���������ʹƷ����ɫ��������������Ƿ������
�ʴ�Ϊ����ȥSO2 ����SO2�Ƿ��ѳ�����
��3��Ҫ���������̼��Ӧ�ų����������ݶ�����̼�����ʯ��ˮ����������������̼��
��Ӧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��
�ʴ�Ϊ��B��Ʒ�첻��ɫ C�в������ǣ����ɫ������
�����������ص㿼���˻�������ж�����̼�ļ��飬һ��Ҫ�ų���������ĸ��ţ��ٽ��м��飬���������ʵ����������

��ϰ��ϵ�д�
�����Ŀ
 д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ______��
д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ______��