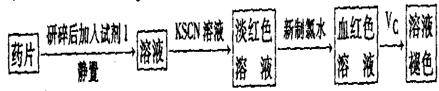
��Ŀ����
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________��
![]()
![]() ��
��
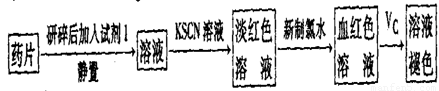
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
��A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc��_______�ԣ�ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ![]() ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
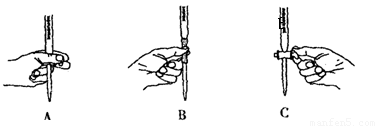
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
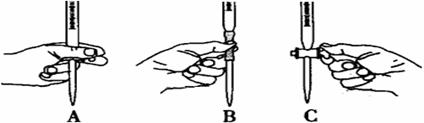
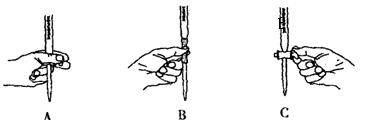
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���
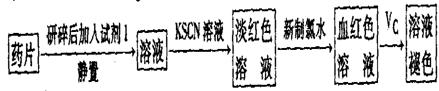
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
��1��![]()
��2��B��C
��3����ԭ��Fe2+������Ѫ�쵰��������O2�����ã�Fe3+û�д˹��ܣ�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+���������ֻ������Fe2+��Fe2+������Ѫ�쵰��������O2�����ã�����Vc�ɷ�ֹҩƷ�е�Fe2+��������Fe3+��
��4��C
��5��A
(6)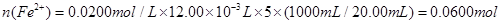
![]()
������������![]()
��ҩƷ�����������ϸ�
����:��

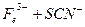

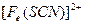 ��
��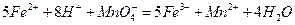 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��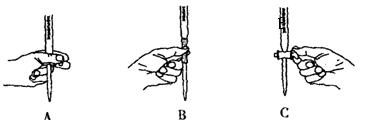
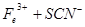
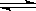
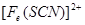 ��
��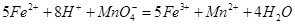 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��