��Ŀ����
��14�֣�
I.��ʵ������������װ�ã����Ʊ�ijЩ���岢��֤�仯ѧ���ʡ�
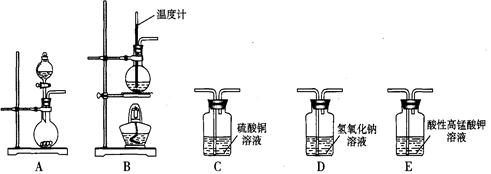
��������
II. ��1����ҵ������ϩ������Ϊԭ�ϣ������и����ϳɾ�����ϩ��PVC����
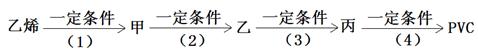
��Ӧ��2���Ļ�ѧ����ʽ��__________________________________________��
PVC�Ľṹ��ʽ��_________________________________________________��
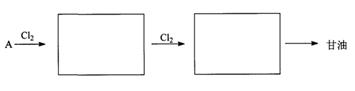
��2��ʯ�ͱ���Ϊ����ҵѪҺ����A�Ǵ�ʯ���еõ�����״������Է�������Ϊ42����AΪԭ�ϣ�����������Ӧ�ϳɸ��ͣ��ڷ����������м���Ľṹ��ʽ��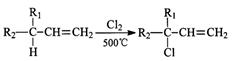
I.��ʵ������������װ�ã����Ʊ�ijЩ���岢��֤�仯ѧ���ʡ�

��������
| ��� | ���� | װ������˳������ĸ�� | �Ʊ���Ӧ�Ļ�ѧ����ʽ |
| ��1�� | ��ϩ | __________________ | _________________________ |
| ��2�� | ��Ȳ | A��C��E | _________________________ |
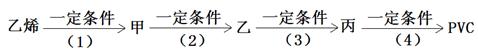
��Ӧ��2���Ļ�ѧ����ʽ��__________________________________________��
PVC�Ľṹ��ʽ��_________________________________________________��
��2��ʯ�ͱ���Ϊ����ҵѪҺ����A�Ǵ�ʯ���еõ�����״������Է�������Ϊ42����AΪԭ�ϣ�����������Ӧ�ϳɸ��ͣ��ڷ����������м���Ľṹ��ʽ��


��14�֣�ÿ��2�֣�
I.��1�� B��D��E
��2��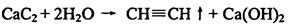
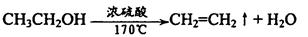
��. ��1��CH2ClCH2Cl+2NaOH �� CH��CH��+2NaCl+2H2O
 ��2��
��2��
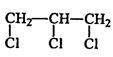
I.��1�� B��D��E

��2��
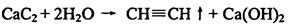
��. ��1��CH2ClCH2Cl+2NaOH �� CH��CH��+2NaCl+2H2O
 ��2��
��2��
��

��ϰ��ϵ�д�
�����Ŀ

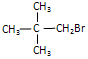
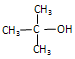
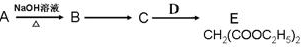
 ��R��R��Ϊ��������ԭ�ӡ���ͼ����E�ϳ�һ�ְͱ���H��һ����Ҫ�л��м��廷����ᣨI�������̣�
��R��R��Ϊ��������ԭ�ӡ���ͼ����E�ϳ�һ�ְͱ���H��һ����Ҫ�л��м��廷����ᣨI�������̣�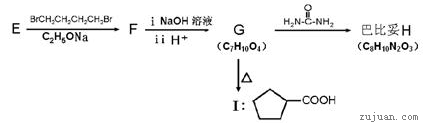
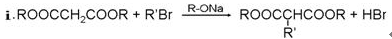
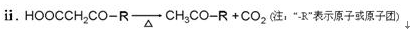
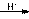 HOCH2NH-CO-NHCH2OH
HOCH2NH-CO-NHCH2OH (HOCH2)2N-CO-N(CH2OH)2
(HOCH2)2N-CO-N(CH2OH)2 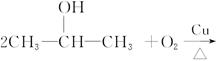
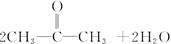
 2CH3CH2CHO+2H2O
2CH3CH2CHO+2H2O C3H7OH+NaX
C3H7OH+NaX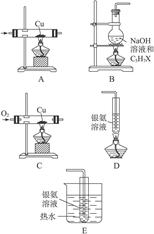
 B�ķ�Ӧ����Ϊ ��Ӧ��C����������Ӧ�Ļ�ѧ����ʽΪ�� ��
B�ķ�Ӧ����Ϊ ��Ӧ��C����������Ӧ�Ļ�ѧ����ʽΪ�� ��