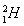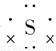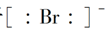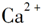ΧβΡΩΡΎ»ί
‘ΣΥΊ÷ήΤΎ±μ «―ßœΑΜ·―ßΒΡ÷Ί“ΣΙΛΨΏΘ§Υϋ“ΰΚ§–μΕύ–≈œΔΚΆΙφ¬…ΓΘœ¬±μΥυΝ– «Έε÷÷ΕΧ÷ήΤΎΒΡ‘≠Ή”ΑκΨΕΦΑ÷ς“ΣΜ·ΚœΦέΘ®“―÷ΣνκΒΡ‘≠Ή”ΑκΨΕΈΣ0.089 nmΘ©ΓΘ
‘ΣΥΊ¥ζΚ≈ABCDE
‘≠Ή”ΑκΨΕ/nm0.160.1430.1020.0990.074
÷ς“ΣΜ·ΚœΦέΘΪ2ΘΪ3ΘΪ6Θ§Θ≠2Θ≠1Θ≠2
Θ®1Θ©”Ο‘ΣΥΊ¥ζΚ≈±ξ≥ωΥϋΟ«‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–Ε‘”ΠΒΡΈΜ÷ΟΘ®“‘œ¬ΈΣ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ©ΓΘ
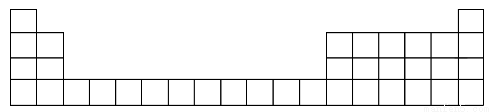
Θ®2Θ©B‘ΣΥΊ¥Π”Ύ÷ήΤΎ±μ÷–ΒΎ__________÷ήΤΎΓΔΒΎ________ΉεΓΘ
Θ®3Θ©BΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο”κCΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______________ΓΘ
Θ®4Θ©…œ ωΈε÷÷‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΥα–‘Ήν«ΩΒΡ «________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®5Θ©CΓΔE–Έ≥…ΒΡΜ·ΚœΈοΈΣ________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®1Θ©
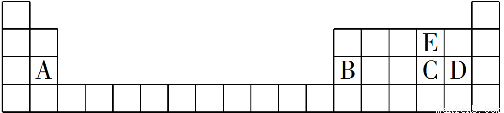
Θ®2Θ©»ΐΓΓΔσA
Θ®3Θ©AlΘ®OHΘ©3ΘΪ3HΘΪ=Al3ΘΪΘΪ3H2O
Θ®4Θ©HClO4
Θ®5Θ©SO2ΓΔSO3
ΓΨΫβΈωΓΩ”…÷ς“ΣΜ·ΚœΦέΚΆ‘≠Ή”ΑκΨΕ÷ΣAΈΣMgΘ§BΈΣAlΘ§CΈΣSΘ§DΈΣClΘ§EΈΣOΘ§‘ρB¥Π”Ύ÷ήΤΎ±μ÷–ΒΎ»ΐ÷ήΤΎΓΔΒΎΔσAΉεΘΜΈε÷÷‘ΣΥΊΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο”–MgΘ®OHΘ©2ΓΔAlΘ®OHΘ©3ΓΔH2SO4ΓΔHClO4Θ§Τδ÷–HClO4Υα–‘Ήν«ΩΘΜS”κO–Έ≥…ΒΡΜ·ΚœΈο”–SO2ΚΆSO3ΓΘ

 ΩΎΥψΧβΩ®Φ””Π”ΟΧβΦ·―ΒœΒΝ–¥πΑΗ
ΩΎΥψΧβΩ®Φ””Π”ΟΧβΦ·―ΒœΒΝ–¥πΑΗ ΉέΚœΉ‘≤βœΒΝ–¥πΑΗ
ΉέΚœΉ‘≤βœΒΝ–¥πΑΗ