��Ŀ����
��14�֣�����ʱ����ϡ��Һ��c(H+)��c(OH-)�ij˻����ǵ��� ��
��1��������Һc(H+) �����������������=������ͬ��c(OH-)��pH 7��
�˵�θҺ�к������ᣬ���ⶨij��θҺ��pHΪ2�������е�c(H+)= mol/L��
ʳ�׳�����ʳƷ��ζ������Ҫ�ɷ�Ϊ���ᣬ����ĵ��뷽��ʽΪ ��
��2�������£�pH��Ϊ3������ʹ�����Һ�����и�������ȵ��� ������ţ���
����Һ��c(H+) ��������ʵ���Ũ�� ����ȫ�к�ʱ������NaOH������
��3���ڢ�NaCl����CH3COONa����NH4Cl��������Һ�У������³����Ե��� ������ţ���ͬ����, �����Ե��� , �ʼ��Ե��� ��
��4���Ȼ���ˮ������ӷ���ʽΪ �������Ȼ�����Һʱ�μ���������������� ��
��1��������Һc(H+) �����������������=������ͬ��c(OH-)��pH 7��
�˵�θҺ�к������ᣬ���ⶨij��θҺ��pHΪ2�������е�c(H+)= mol/L��
ʳ�׳�����ʳƷ��ζ������Ҫ�ɷ�Ϊ���ᣬ����ĵ��뷽��ʽΪ ��
��2�������£�pH��Ϊ3������ʹ�����Һ�����и�������ȵ��� ������ţ���
����Һ��c(H+) ��������ʵ���Ũ�� ����ȫ�к�ʱ������NaOH������
��3���ڢ�NaCl����CH3COONa����NH4Cl��������Һ�У������³����Ե��� ������ţ���ͬ����, �����Ե��� , �ʼ��Ե��� ��
��4���Ȼ���ˮ������ӷ���ʽΪ �������Ȼ�����Һʱ�μ���������������� ��
��14�֣�1��10-14
��1���� �� 0.01 CH3COOH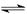 CH3COO-+H+
CH3COO-+H+
��2���� ��3���� �� ��
��4��Fe3++3H2O Fe(OH)3+3H+ ����Fe3+ˮ��
Fe(OH)3+3H+ ����Fe3+ˮ��
��1���� �� 0.01 CH3COOH
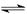 CH3COO-+H+
CH3COO-+H+��2���� ��3���� �� ��
��4��Fe3++3H2O
 Fe(OH)3+3H+ ����Fe3+ˮ��
Fe(OH)3+3H+ ����Fe3+ˮ���������������ʱ����ϡ��Һ��c(H+)��c(OH-)�ij˻������ǵ����ӻ���������ֵ����1��10-14��
��1��������������Һ��������Ũ���Ǵ���c(OH-)����ʱpH����7��������Ũ�ȵĸ�������pH��������pH��2������£�c(H+)=0.01mol/L�����������ᣬ���ֵ��룬���뷽��ʽ��
CH3COOH
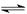 CH3COO-+H+��
CH3COO-+H+����2������������ǿ�ᣬ�����������ᣬ������pH��Ϊ3������ʹ�����Һ�У�������Ũ������ȵģ��������Ũ�ȴ�������ģ����Դ������ĵ��������ƶ࣬��ѡ�١�
��3���Ȼ�����ǿ��ǿ���Σ���ˮ�⣬��Һ�����ԣ���������ǿ�������Σ�ˮ���Լ��ԣ��Ȼ����ǿ�������Σ�ˮ�������ԡ�
��4���Ȼ���Ҳ��ǿ�������Σ�������ˮ�⣬�����������������ᣬ���������Ȼ�����Һʱ�μ�������������������������ӵ�ˮ�⡣
���������ж���Һ����ԣ�����ֱ�Ӹ���pH��7�Ĵ�С���бȽϣ���Ϊֻ���ڳ��³�ѹ�£����Ƿ��ϸù�ϵʽ�ġ��������Ӻ�OH��Ũ�ȵ���Դ�С�����κ������¶��dz����ġ�

��ϰ��ϵ�д�
�����Ŀ
 ��
��