��Ŀ����
��A��B��C��D���ֶ�����Ԫ��, ���ǵ�ԭ��������A��D��������, ��֪A��Bԭ������ͬ�ĵ��Ӳ���, ��A��L���������K�������������, C�ڿ�����ȼ��ʱ���ֻ�ɫ����, C�ĵ����ڼ�������B�ĵ��ʳ�ַ�Ӧ, ���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������, �Ը������������ش�:
(1) Ԫ������: B ______ �� D ______��
(2) CԪ��λ�����ڱ��� ���� �塣
(3) д��A������������������C������������ˮ���ﷴӦ�����ӷ���ʽ��
__________________________________________________________________________��
(4) �õ���ʽ��ʾ������ C2D ���γɹ��̣�
__________________________________________________________________________��
(1) Ԫ������: B ______ �� D ______��
(2) CԪ��λ�����ڱ��� ���� �塣
(3) д��A������������������C������������ˮ���ﷴӦ�����ӷ���ʽ��
__________________________________________________________________________��
(4) �õ���ʽ��ʾ������ C2D ���γɹ��̣�
__________________________________________________________________________��
��12�֣�(1) �� �� (2) ���� ��A (3) CO2 + 2OH����CO32��+H2O
(4)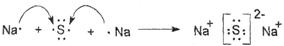
(4)
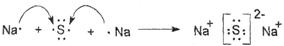
�����������1��A��L���������K�������������������A��̼Ԫ�ء�C�ڿ�����ȼ��ʱ���ֻ�ɫ���棬����C����Ԫ�ء�C�ĵ����ڼ�������B�ĵ��ʳ�ַ�Ӧ, ���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬�������A��Bԭ������ͬ�ĵ��Ӳ���������B����Ԫ�أ�D����Ԫ�ء�
��2����Ԫ�ص�ԭ��������11������λ�ڵ������ڵ�IA�塣
��3��CO2����������������Һ��Ӧ����̼���ƺ�ˮ����Ӧ�����ӷ���ʽ��CO2 + 2OH����CO32��+H2O��
��4�������Ǻ������Ӽ������ӻ�������γɹ�����
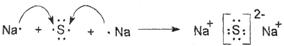 ��
�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ���������

��ϰ��ϵ�д�
�����Ŀ

 .�� ��
.�� ��