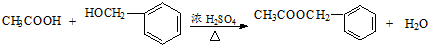��Ŀ����
��������һ�������˿ڵĽ�����裮���������ijɷ��ж��֣����ᱽ������
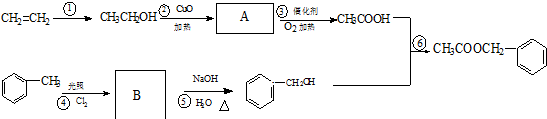
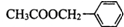 �����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
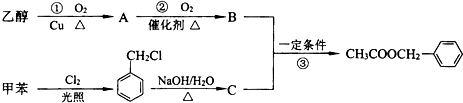
��1��д����Ӧ�ٵĻ�ѧ����ʽ��
��2����Ӧ�۵ķ�Ӧ����Ϊ
��3����Ӧ
��4��C�Ľṹ��ʽΪ
 Cͨ�������ֲ�ͬ���ķ�����ͬ���칹�壬��д�������ֲ�ͬ����ͬ���칹��Ľṹ��ʽ����дһ�֣�
Cͨ�������ֲ�ͬ���ķ�����ͬ���칹�壬��д�������ֲ�ͬ����ͬ���칹��Ľṹ��ʽ����дһ�֣�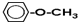 ��
��
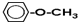 ��
�� ��
��
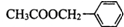 �����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
��1��д����Ӧ�ٵĻ�ѧ����ʽ��
2CH3CH2OH+O2
2CH3CHO+2H2O
| Cu |
| �� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| Cu |
| �� |
��2����Ӧ�۵ķ�Ӧ����Ϊ
ȡ����������
ȡ����������
����3����Ӧ
��
��
������ţ�ԭ�ӵ�����������Ϊ100%��������ɫ��ѧ��Ҫ����4��C�Ľṹ��ʽΪ


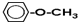 ��
��
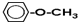 ��
��
������CH3CH2OH��Cu��Ag�������������¿ɱ�����ΪCH3CHO��CH3CHO��һ������������CH3COOH���ױ��ڹ�������������������ȡ����Ӧ����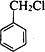 ���ڼ���������ˮ������
���ڼ���������ˮ������ ��
�� ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������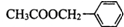 ���Դ˽����⣮
���Դ˽����⣮
 ���ڼ���������ˮ������
���ڼ���������ˮ������ ��
�� ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������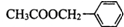 ���Դ˽����⣮
���Դ˽����⣮����⣺CH3CH2OH��Cu��Ag�������������¿ɱ�����ΪCH3CHO��CH3CHO��һ������������CH3COOH���ױ��ڹ�������������������ȡ����Ӧ���� ���ڼ���������ˮ������
���ڼ���������ˮ������ ��
�� ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������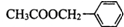 ��
��
��1����Ӧ��ΪCH3CH2OH��Cu��Ag�������������¿ɱ�����ΪCH3CHO�ķ�Ӧ��
��ѧ����ʽΪ2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��
��2����Ӧ��Ϊ ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������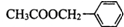 ��ҲΪȡ����Ӧ���ʴ�Ϊ��ȡ������������
��ҲΪȡ����Ӧ���ʴ�Ϊ��ȡ������������
��3����Ӧ�٢۶���ˮ���ɣ���Ӧ��Ϊ2CH3CHO+O2��2CH3COOH��ԭ�ӵ�����������Ϊ100%��������ɫ��ѧ��Ҫ��
�ʴ�Ϊ���ڣ�
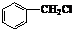
��4��CΪ ����Ӧ��ͬ���칹���Ϊ�������࣬��
����Ӧ��ͬ���칹���Ϊ�������࣬��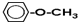 ��
�� �ȣ�
�ȣ�
�ʴ�Ϊ�� ��
��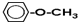 ��
�� ��
��
 ���ڼ���������ˮ������
���ڼ���������ˮ������ ��
�� ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������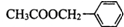 ��
����1����Ӧ��ΪCH3CH2OH��Cu��Ag�������������¿ɱ�����ΪCH3CHO�ķ�Ӧ��
��ѧ����ʽΪ2CH3CH2OH+O2
| Cu |
| �� |
| Cu |
| �� |
��2����Ӧ��Ϊ
 ��CH3COOH��һ�������·���������Ӧ������
��CH3COOH��һ�������·���������Ӧ������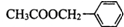 ��ҲΪȡ����Ӧ���ʴ�Ϊ��ȡ������������
��ҲΪȡ����Ӧ���ʴ�Ϊ��ȡ��������������3����Ӧ�٢۶���ˮ���ɣ���Ӧ��Ϊ2CH3CHO+O2��2CH3COOH��ԭ�ӵ�����������Ϊ100%��������ɫ��ѧ��Ҫ��
�ʴ�Ϊ���ڣ�
��4��CΪ
 ����Ӧ��ͬ���칹���Ϊ�������࣬��
����Ӧ��ͬ���칹���Ϊ�������࣬��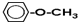 ��
�� �ȣ�
�ȣ��ʴ�Ϊ��
 ��
��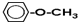 ��
�� ��
�����������⿼���л���ĺϳɣ���Ŀ�Ѷ��еȣ��漰���ʶ�Ϊ��ѧ�г������ʣ�ע������л���Ĺ����ŵĽṹ�����ʣ�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ

 ��X���ܵĽṹ��
��X���ܵĽṹ�� �������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·���磺
�������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·���磺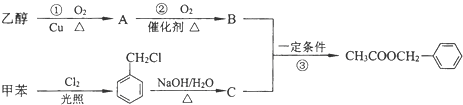

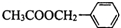 �������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
�������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�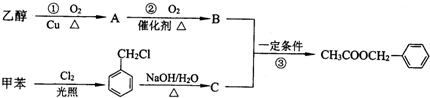


 �������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�
�������е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɣ�һ�ֺϳ�·�����£�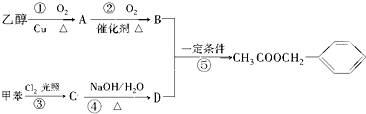
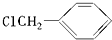
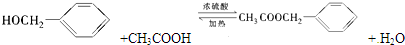
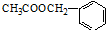 ���е�һ�֣������Դ���������ȡ��Ҳ������ϩ�ͼױ�Ϊԭ�Ͻ����˹��ϳɣ�����һ�ֺϳ�·�����£�
���е�һ�֣������Դ���������ȡ��Ҳ������ϩ�ͼױ�Ϊԭ�Ͻ����˹��ϳɣ�����һ�ֺϳ�·�����£�