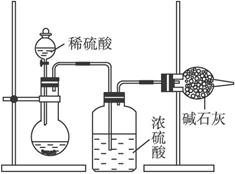
��Ŀ����
�ⶨ��Ʒ�гɷֺ�����ʵ��һ��Ӧ�ظ��������Σ�Ϊ�˲ⶨijNaOH�����л��е�Na2CO3������������ijͬѧ�ֱ����������ʵ�鷽����I�����շ�
��ͼ��ʾ��
��1������ʵ��װ��ͼ��������ÿ��ʵ������������еij�����������Ҫ����______�Σ��ҵ�һ�γ��������Ķ�����______��
��2�����ظ��ⶨ�����Σ��õ�Na2CO3���������������ݴ��ڽϴ��ƫ�����ƫ���ԭ�������______������ţ���
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B������ϡ��������Ʒ��Ӧ
C������ϡ����������㣬�����������
D����Ӧ��ɺ�װ�����Դ��ж�����̼��û�б���ʯ������
������ԭ���⣬��װ�ÿ�����һ����Ҫԭ����______��
II�����
��ȡ��Ʒm g�����Ƴ�500mL��Һ������ȡ��25mL����������BaCl2��Һ���μ�2��3�η�̪����a mol/L������ζ������յ�ʱ��������v mL��
��1����ͬѧ����������BaCl2��Һ��δ��BaCO3���˾͵ζ���������Ϊ�ڵζ���BaCO3��ת��ΪBa��HCO3��2���������ᣮ����ΪҪ������______����ǡ����������ǣ�______������ǡ�������𣩣�
��2���жϵζ��յ㵽���������______��
������
��ȡ��Ʒm g�����ܽ�ӹ���Ba��NO3��2��Һ�����ˡ�ϴ�ӡ���ɣ������ù���n g��
��1���������̼���Ƶ���������Ϊ����m��n��ʾ��______��
��2��ϴ�ӳ����IJ���Ҫ����______��
��3��Ca2+��Ba2+������ʹCO32-������ȫ����ʹ��Ba��NO3��2��Һ������Ca��NO3��2��Һ��ԭ����______���ⶨCO32-��������ʹ��Ba��OH��2��Һ������Ca��OH��2��Һ����������и��ߵľ�ȷ�ȣ�ԭ��______��
���𰸡���������1������ʵ��Ŀ���Dzⶨ̼��������������ʵ�鷽����Ҫ�ⶨ���ɶ�����̼�������������õ���ʵ����Ҫ����������ƿ��������������Ʒ����ƿ��������װ�м�ʯ�ҵĸ������������Ӧ������������
��2��A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ�����ս��ƫ��
B������ϡ��������Ʒ��Ӧ�Խ����Ӱ�죻
C������ϡ����������㣬����������ʣ��̼���ƣ��ⶨ���ƫС��
D����Ӧ��ɺ�װ�����Դ��ж�����̼��û�б���ʯ�����գ����ɵĶ�����̼����ȫ�����ս��ƫ�ͣ�
��1�����Ի����·�̪��ɫָʾ���յ㣬̼�ᱵ�ڼ��������²���Ӧ��
��2��ϴ�ӲⶨӦ�ڹ���װ���н��У�
��3��������������ˮ��ʹ��Һ����ǣ���̼���������һ���������³���̼�ᱵ��̼��Ƹ�ȷ��
����⣺��1������ʵ��Ŀ���Dzⶨ̼��������������ʵ�鷽����Ҫ�ⶨ���ɶ�����̼�������������õ���ʵ����Ҫ����������ƿ��������������Ʒ����ƿ��������װ�м�ʯ�ҵĸ������������Ӧ���������������Բⶨ������Ҫ����4�Σ���һ����Ҫ����������ƿ������������ȷ����������Ʒ���������ʴ�Ϊ��4������������ƿ��������
��2�����ظ��ⶨ�����Σ��õ�Na2CO3���������������ݴ��ڽϴ��ƫ�����ƫ���ԭ��
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ�����ս��ƫ��A���ϣ�
B������ϡ��������Ʒ��Ӧ�Խ����Ӱ�죬��B�����ϣ�
C������ϡ����������㣬����������ʣ��̼���ƣ��ⶨ���ƫС����C���ϣ�
D����Ӧ��ɺ�װ�����Դ��ж�����̼��û�б���ʯ�����գ����ɵĶ�����̼����ȫ�����ս��ƫ�ͣ���D���ϣ�
�������ܺͿ���ֱ�ӽӴ��������տ����еĶ�����̼��ʵ��ⶨ������
�ʴ�Ϊ��ACD��װ����Ŀ����е�ˮ�����Ͷ�����̼����ʯ�����գ�
��1������������BaCl2��Һ��δ��BaCO3���˾͵ζ���������������Ʒ�Ӧ���̼���Ʒ�Ӧ��ȫ���յ���ҺPH��8-10���������ԣ���ʱ̼�ᱵ���������ܽ⣬
�ʴ�Ϊ�����÷�̪��ָʾ�����յ�ʱ��Һ�����ļ��ԣ�BaCO3����Ӧ��
��2��ϴ�ӲⶨӦ�ڹ���װ���н��У�����Ҫ���ǣ��ò�������������еij�����ˮ����û������ʹˮ�˳������ظ�2-3�Σ�
�ʴ�Ϊ���ò�������������еij�����ˮ����û������ʹˮ�˳������ظ�2-3�Σ�
��3��������������������������������ˮ���������Ƴ�����Ӱ��ⶨ�����������������ˮ��ʹ��Һ����ǣ���̼������ӵ���һ����������̼�ᱵ��̼��Ƹ�ȷ��̼�ᱵ��Է�����������̼��ƣ���̼��������ƶ������£���������������Һ���ɳ����࣬�������������С���ʴ�Ϊ��������������ˮ��BaCO3��Է���������������С��
���������⿼�������ʺ����ⶨ��ʵ��Ӧ�ã�ʵ�鷽������ƣ��Լ���ѡ���������ǽ���ؼ�����Ŀ�Ѷ��еȣ�
��2��A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ�����ս��ƫ��
B������ϡ��������Ʒ��Ӧ�Խ����Ӱ�죻
C������ϡ����������㣬����������ʣ��̼���ƣ��ⶨ���ƫС��
D����Ӧ��ɺ�װ�����Դ��ж�����̼��û�б���ʯ�����գ����ɵĶ�����̼����ȫ�����ս��ƫ�ͣ�
��1�����Ի����·�̪��ɫָʾ���յ㣬̼�ᱵ�ڼ��������²���Ӧ��
��2��ϴ�ӲⶨӦ�ڹ���װ���н��У�
��3��������������ˮ��ʹ��Һ����ǣ���̼���������һ���������³���̼�ᱵ��̼��Ƹ�ȷ��
����⣺��1������ʵ��Ŀ���Dzⶨ̼��������������ʵ�鷽����Ҫ�ⶨ���ɶ�����̼�������������õ���ʵ����Ҫ����������ƿ��������������Ʒ����ƿ��������װ�м�ʯ�ҵĸ������������Ӧ���������������Բⶨ������Ҫ����4�Σ���һ����Ҫ����������ƿ������������ȷ����������Ʒ���������ʴ�Ϊ��4������������ƿ��������
��2�����ظ��ⶨ�����Σ��õ�Na2CO3���������������ݴ��ڽϴ��ƫ�����ƫ���ԭ��
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ�����ս��ƫ��A���ϣ�
B������ϡ��������Ʒ��Ӧ�Խ����Ӱ�죬��B�����ϣ�
C������ϡ����������㣬����������ʣ��̼���ƣ��ⶨ���ƫС����C���ϣ�
D����Ӧ��ɺ�װ�����Դ��ж�����̼��û�б���ʯ�����գ����ɵĶ�����̼����ȫ�����ս��ƫ�ͣ���D���ϣ�
�������ܺͿ���ֱ�ӽӴ��������տ����еĶ�����̼��ʵ��ⶨ������
�ʴ�Ϊ��ACD��װ����Ŀ����е�ˮ�����Ͷ�����̼����ʯ�����գ�
��1������������BaCl2��Һ��δ��BaCO3���˾͵ζ���������������Ʒ�Ӧ���̼���Ʒ�Ӧ��ȫ���յ���ҺPH��8-10���������ԣ���ʱ̼�ᱵ���������ܽ⣬
�ʴ�Ϊ�����÷�̪��ָʾ�����յ�ʱ��Һ�����ļ��ԣ�BaCO3����Ӧ��
��2��ϴ�ӲⶨӦ�ڹ���װ���н��У�����Ҫ���ǣ��ò�������������еij�����ˮ����û������ʹˮ�˳������ظ�2-3�Σ�
�ʴ�Ϊ���ò�������������еij�����ˮ����û������ʹˮ�˳������ظ�2-3�Σ�
��3��������������������������������ˮ���������Ƴ�����Ӱ��ⶨ�����������������ˮ��ʹ��Һ����ǣ���̼������ӵ���һ����������̼�ᱵ��̼��Ƹ�ȷ��̼�ᱵ��Է�����������̼��ƣ���̼��������ƶ������£���������������Һ���ɳ����࣬�������������С���ʴ�Ϊ��������������ˮ��BaCO3��Է���������������С��
���������⿼�������ʺ����ⶨ��ʵ��Ӧ�ã�ʵ�鷽������ƣ��Լ���ѡ���������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
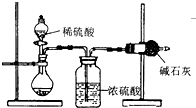 �ⶨ��Ʒ�гɷֺ�����ʵ��һ��Ӧ�ظ��������Σ�Ϊ�˲ⶨijNaOH�����л��е�Na2CO3������������ijͬѧ�ֱ����������ʵ�鷽����
�ⶨ��Ʒ�гɷֺ�����ʵ��һ��Ӧ�ظ��������Σ�Ϊ�˲ⶨijNaOH�����л��е�Na2CO3������������ijͬѧ�ֱ����������ʵ�鷽����