��Ŀ����
��10�֣�����Ԫ��A��B��C��D��Eλ��Ԫ�����ڱ���ǰ�����ڣ���֪���ǵĺ˵�����������ӣ�A�������к�������Ԫ�أ�Bԭ�ӵ�L��p�������2�����ӣ�C��Bԭ�ӵļ۲��������ͬ��Dԭ��M���d�����һ���ɶԵ��ӣ�Eԭ�ӵ�L�������������������֮��Ϊ4:1����d����еĵ�����������������֮��Ϊ5:1��
��1��B��A�γ�ֻ��һ������ԭ�ӵĹ��ۻ��������ӵĵ���ʽΪ ������ԭ�ӵ��ӻ���������� ������ӵĵ����幹����
��2��A����������B���������У����Ӽ��Խ�С���ǣ������ʽ��__________
��3��B��C�Ƚϣ��縺�Խϴ�ģ���Ԫ�����ƣ�____________
��4��д��D�ļ۲�����Ų�ʽ
��5��E��Al�Ļ�ѧ�������ƣ���д��E��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
��6��д��D����������������ᷴӦ�����ӷ���ʽ
��1��B��A�γ�ֻ��һ������ԭ�ӵĹ��ۻ��������ӵĵ���ʽΪ ������ԭ�ӵ��ӻ���������� ������ӵĵ����幹����
��2��A����������B���������У����Ӽ��Խ�С���ǣ������ʽ��__________
��3��B��C�Ƚϣ��縺�Խϴ�ģ���Ԫ�����ƣ�____________
��4��д��D�ļ۲�����Ų�ʽ
��5��E��Al�Ļ�ѧ�������ƣ���д��E��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
��6��д��D����������������ᷴӦ�����ӷ���ʽ
��10�֣���1������ʽ ������ sp3�ӻ� �����幹���� ��������
��2�����Ӽ��Խ�С���ǣ������ʽ��_CO2__ ��3���縺�Խϴ�ģ���Ԫ�����ƣ�___̼__
��4���۲�����Ų�ʽ 3d64s2
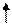 ��5��ѧ����ʽ Zn + 2NaOH = Na2ZnO2 + H2
��5��ѧ����ʽ Zn + 2NaOH = Na2ZnO2 + H2
��6�����ӷ���ʽ Fe2O3 + 6H+ = 2Fe3+ + 3H2O
��2�����Ӽ��Խ�С���ǣ������ʽ��_CO2__ ��3���縺�Խϴ�ģ���Ԫ�����ƣ�___̼__
��4���۲�����Ų�ʽ 3d64s2
 ��5��ѧ����ʽ Zn + 2NaOH = Na2ZnO2 + H2
��5��ѧ����ʽ Zn + 2NaOH = Na2ZnO2 + H2 ��6�����ӷ���ʽ Fe2O3 + 6H+ = 2Fe3+ + 3H2O
��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д�
�����Ŀ
 ��1��Xԭ�ӵ��۵����Ų�ʽ�ǣ�______________________________________��
��1��Xԭ�ӵ��۵����Ų�ʽ�ǣ�______________________________________�� �е�λ�ù�ϵ��ͼ��ʾ��������������ȷ���ǣ� ��
�е�λ�ù�ϵ��ͼ��ʾ��������������ȷ���ǣ� ��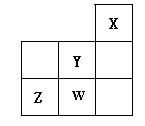
 Һ��Ӧʱ��ת�Ƶĵ�����Ϊ1mol
Һ��Ӧʱ��ת�Ƶĵ�����Ϊ1mol