��Ŀ����
��2013?����һģ����ҵ����K3[Fe��C2O4��3]?3H2O�Ǵ���ɫ���壬��421��553��ʱ���ֽ�ΪFe2O3��K2CO3��CO��CO2��H2O��ʵ�����ɲ����������壨FeC2O4?2H2O��������أ�K2C2O4�������ᣨH2C2O4����˫��ˮ��H2O2������Ʊ���
��ش��������⣺
��1��д��H2O2�ĵ���ʽ
 ��[Fe��C2O4��3]3-��������
��[Fe��C2O4��3]3-��������
��2����ƽ�����ܷ�Ӧ����ʽ��
��3���Ʊ�������Ҫ��ֹ���ᱻH2O2��������д�����ᱻH2O2�����Ļ�ѧ��Ӧ����ʽ
��4���������ȶ��Կ������ȶ�����K����������Cu2++4NH3?[Cu��NH3��4]2+�����ȶ���������ʽΪk=
����֪K[Fe��C2O4��3]3-=1020��K[Fe��SCN��3]=2��103���ܷ���KSCN��Һ����K3[Fe��C2O4��3]?3H2O�е���Ԫ�أ�
��5����Ԫ�ؿ����γɶ�����������һ����������A���������ʳƷ���Ӽ�������ɷ���A�н���K��Fe��C��N����Ԫ�أ�ȡ36.8gA������400�棬�ֽ��KCN��Fe3C��C��N2�����ɵĵ����ۺϳɱ�״���µ����Ϊ2.24L��Fe3C������C������3����Fe3C�����ʵ����ǵ������ʵ�����
����A�Ļ�ѧʽΪ
��ش��������⣺
��1��д��H2O2�ĵ���ʽ


[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�����������������
[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�����������������
����2����ƽ�����ܷ�Ӧ����ʽ��
2
2
FeC2O4?2H2O+1
1
H2O2+3
3
K2C2O4+1
1
H2C2O4=3
3
K3[Fe��C2O4��3]?3H2O��3���Ʊ�������Ҫ��ֹ���ᱻH2O2��������д�����ᱻH2O2�����Ļ�ѧ��Ӧ����ʽ
H2C2O4+H2O2=2CO2��+2H2O
H2C2O4+H2O2=2CO2��+2H2O
����4���������ȶ��Կ������ȶ�����K����������Cu2++4NH3?[Cu��NH3��4]2+�����ȶ���������ʽΪk=
c[Cu(NH3
| ||
| c(Cu2+)?c4(NH3) |
��
��
����ǡ�������ѡ��������Ƽ�����Ԫ�صķ���ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮����
ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮����
����5����Ԫ�ؿ����γɶ�����������һ����������A���������ʳƷ���Ӽ�������ɷ���A�н���K��Fe��C��N����Ԫ�أ�ȡ36.8gA������400�棬�ֽ��KCN��Fe3C��C��N2�����ɵĵ����ۺϳɱ�״���µ����Ϊ2.24L��Fe3C������C������3����Fe3C�����ʵ����ǵ������ʵ�����
| 1 |
| 3 |
K4Fe��CN��6
K4Fe��CN��6
����������1��H2O2Ϊ���ۻ����[Fe��C2O4��3]3-Ϊ�����ӣ�
��2������ԭ���غ�����ƽ��ѧ��Ӧ����ʽ��
��3�����ᱻH2O2��������ˮ�Ͷ�����̼��
��4��K3[Fe��C2O4��3]?3H2O�е���Ϊ�������ӣ�����������KSCN��Һ��ΪѪ��ɫ��
��5��A��KCN+Fe3C+C+N2��n��N2��=0.1mol��n��Fe3C��=0.1mol��
��n��C��=
=
mol��n��KCN��=
=0.4mol��ȷ��ԭ�Ӹ����ȵó���ѧʽ��
��2������ԭ���غ�����ƽ��ѧ��Ӧ����ʽ��
��3�����ᱻH2O2��������ˮ�Ͷ�����̼��
��4��K3[Fe��C2O4��3]?3H2O�е���Ϊ�������ӣ�����������KSCN��Һ��ΪѪ��ɫ��
��5��A��KCN+Fe3C+C+N2��n��N2��=0.1mol��n��Fe3C��=0.1mol��
| 1 |
| 3 |
0.1mol��
| ||||
| 12g/mol |
| 1 |
| 6 |
| 36.8g-0.1mol��28g/mol-6g-2g |
| 65g/mol |
����⣺��1��H2O2Ϊ���ۻ���������ʽΪ ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ��
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ�� ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�������������������
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�������������������
��2������ˮ�ĸ�������С������Ϊ6����FeC2O4?2H2O�Ļ�ѧ������Ϊ2��K3[Fe��C2O4��3]?3H2O�Ļ�ѧ������Ϊ3��Ȼ����ԭ���غ��֪���û�ѧ��ӦΪ2FeC2O4?2H2O+H2O2+3K2C2O4+H2C2O4=2K3[Fe��C2O4��3]?3H2O���ʴ�Ϊ��2��1��3��1��2��
��3�����ᱻH2O2��������ˮ�Ͷ�����̼���÷�ӦΪH2C2O4+H2O2=2CO2��+2H2O���ʴ�Ϊ��H2C2O4+H2O2=2CO2��+2H2O��
��4��K3[Fe��C2O4��3]?3H2O�е���Ϊ�������ӣ�������KSCN��Һ����K3[Fe��C2O4��3]?3H2O�е���Ԫ�أ�������Ԫ�صķ���Ϊȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮���ޣ�
�ʴ�Ϊ����ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮���ޣ�
��5���������֪��A��KCN+Fe3C+C+N2��n��N2��=0.1mol��n��Fe3C��=0.1mol��
��n��C��=
=
mol��n��KCN��=
=0.4mol����K��Fe��C��N��ԭ�Ӹ�����Ϊ4��1��6��6����ѧʽΪK4Fe��CN��6���ʴ�Ϊ��K4Fe��CN��6��
 ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ��
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ��������������������ʴ�Ϊ�� ��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ�������������������
��[Fe��C2O4��3]3-Ϊ�����ӣ�����Ϊ����������������ӣ���������������ӣ���������������������2������ˮ�ĸ�������С������Ϊ6����FeC2O4?2H2O�Ļ�ѧ������Ϊ2��K3[Fe��C2O4��3]?3H2O�Ļ�ѧ������Ϊ3��Ȼ����ԭ���غ��֪���û�ѧ��ӦΪ2FeC2O4?2H2O+H2O2+3K2C2O4+H2C2O4=2K3[Fe��C2O4��3]?3H2O���ʴ�Ϊ��2��1��3��1��2��
��3�����ᱻH2O2��������ˮ�Ͷ�����̼���÷�ӦΪH2C2O4+H2O2=2CO2��+2H2O���ʴ�Ϊ��H2C2O4+H2O2=2CO2��+2H2O��
��4��K3[Fe��C2O4��3]?3H2O�е���Ϊ�������ӣ�������KSCN��Һ����K3[Fe��C2O4��3]?3H2O�е���Ԫ�أ�������Ԫ�صķ���Ϊȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮���ޣ�
�ʴ�Ϊ����ȡ����������ȣ�ȡ����������ܽ���H2SO4�У�ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ������Һ��Ѫ��ɫ������Ԫ�أ���֮���ޣ�
��5���������֪��A��KCN+Fe3C+C+N2��n��N2��=0.1mol��n��Fe3C��=0.1mol��
| 1 |
| 3 |
0.1mol��
| ||||
| 12g/mol |
| 1 |
| 6 |
| 36.8g-0.1mol��28g/mol-6g-2g |
| 65g/mol |
���������⿼��֪ʶ��϶࣬�漰����ʽ����Ӧ����ƽ��������ԭ��Ӧ�����ӵļ��鼰��ѧʽ��ȷ������5����ע�������غ��������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
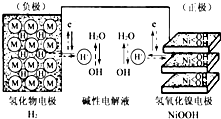 ��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������
��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������