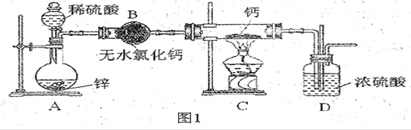
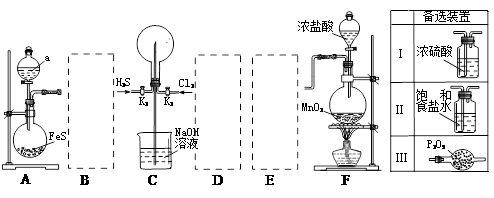
��Ŀ����
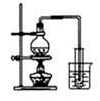
Cl2��H2O2�Ǹ��н����������������������������Cl2��������ǿ��H2O2���ܽ�H2O2������Ϊ����֤�ý��ۣ�ѧ�����������ͼ��ʾ��ʵ��װ�ý���ʵ�飨�г�װ����ȥ����Բ����ƿA�еķ�Ӧ����ʽΪ2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O����ش���������
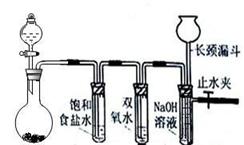
A B C D
��1���Թ�B�б���ʳ��ˮ������ ��
��2���Թ�C�м���5mL 30% ˫��ˮ��������Ӧ�Ļ�ѧ����ʽ____________________��
��3���Թ�D�г���10% NaOH��Һ��NaOH��Һ�������� ��
��4����Ũ����ע��Բ����ƿA����װ���еĿ��������ž���ر�ֹˮ�У���Ӧһ��ʱ����Թ�D�е�����Ϊ ���Թ�D�е�������鷽��Ϊ__________________��
��5���е�ͬѧ������ʵ�����Թ�D��������Դ�������ɣ�����Ϊ���ܵ���Դ�� ���û�ѧ����ʽ��ʾ�����������ɿ��Բ��öԱ�ʵ���������

A B C D
��1���Թ�B�б���ʳ��ˮ������ ��
��2���Թ�C�м���5mL 30% ˫��ˮ��������Ӧ�Ļ�ѧ����ʽ____________________��
��3���Թ�D�г���10% NaOH��Һ��NaOH��Һ�������� ��
��4����Ũ����ע��Բ����ƿA����װ���еĿ��������ž���ر�ֹˮ�У���Ӧһ��ʱ����Թ�D�е�����Ϊ ���Թ�D�е�������鷽��Ϊ__________________��
��5���е�ͬѧ������ʵ�����Թ�D��������Դ�������ɣ�����Ϊ���ܵ���Դ�� ���û�ѧ����ʽ��ʾ�����������ɿ��Բ��öԱ�ʵ���������
��1����ȥCl2�л��е�HCl����2��Cl2+H2O2=2HCl+O2����3�����ն����Cl2����4���Թ�D��Һ���½�������©����Һ����������������ʹ�����ǵ�ľ����ȼ��֤������������5��2H2O2=2H2O+O2����Cl2+H2O="HCl+HClO" ��2HClO=2HCl+O2����
�����������1���Ȼ��⼫������ˮ������������ˮ������ˮ��Ӧ��Cl2+H2O=H++Cl-+HClO��ʳ��ˮ��Һ�е������������������ܽ⣬�����������ܽ�ȣ��ʱ���ʳ��ˮ�������dz�ȥCl2�л��е�HCl����2��Cl2��������ǿ��H2O2���ܽ�H2O2��������O2����ѧ����ʽCl2+H2O2=2HCl+O2��3����������Cl2û�з�����Ӧ�ų�����NaOH��Һ���ն����Cl2��4����Ũ����ע��Բ����ƿ���������������������˫��ˮ��Ӧ���ɴ�����������D��װ���п����Ž��ֹˮ�йرգ�Dװ����ѹǿ����ʹҺ���½�������©����Һ�����������������ķ�����ʹ�����ǵ�ľ����ȼ��5��2H2O2=2H2O+O2����Cl2+H2O=HCl+HClO��2HClO=2HCl+O2����

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ