��Ŀ����
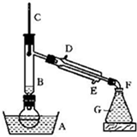
��1��д��ͼ1����Ţ١������������ƣ�
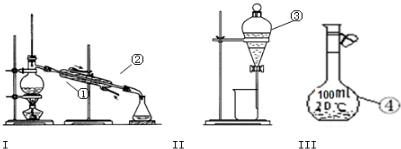
��______��______��______��______
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����______������������ţ�
��3�������ˮ�еĵ�Ӧ��ѡ��װ��______����װ����ţ����в���A����ѡװ�ý���______��������������ƣ������в���Aʱ�����ڵ�ˮ�м�һ�Լ�����ѡ���Լ�ʱ��������������Ϊ��Щ�����DZ���ģ�______������ţ���
�ٳ�����ΪҺ̬��I2�������ܽ�̶ȴ����ˮ���̶ܳ�С���ܶ�Ҫ��ˮ��
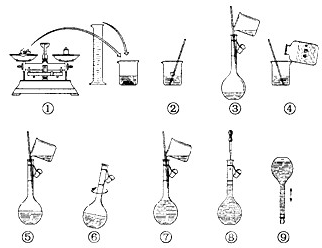
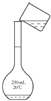
��4��ͼ2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������480mL1mol/L��ϡ���ᣮ
�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
a������ϡ����ʱ����ȱ�ٵ�������______��______��д�������ƣ���
b�������㣬����480mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ______mL����ȡ����ʱӦѡ��______mL������Ͳ��
A��10mLB��50mLC��100mLD��200mL
c���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1mol/L�����ƹ��������и�������������������ԭ����______��
A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B������ʱ����������ƿ�̶��߽��ж���
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D��ת����Һʱ��������������Һ��������ƿ����
E������ƿ������ˮϴ�Ӻ�δ���������������ˮ
F�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���

��______��______��______��______
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ����______������������ţ�
��3�������ˮ�еĵ�Ӧ��ѡ��װ��______����װ����ţ����в���A����ѡװ�ý���______��������������ƣ������в���Aʱ�����ڵ�ˮ�м�һ�Լ�����ѡ���Լ�ʱ��������������Ϊ��Щ�����DZ���ģ�______������ţ���
�ٳ�����ΪҺ̬��I2�������ܽ�̶ȴ����ˮ���̶ܳ�С���ܶ�Ҫ��ˮ��
��4��ͼ2��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������480mL1mol/L��ϡ���ᣮ

�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
a������ϡ����ʱ����ȱ�ٵ�������______��______��д�������ƣ���
b�������㣬����480mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ______mL����ȡ����ʱӦѡ��______mL������Ͳ��
A��10mLB��50mLC��100mLD��200mL
c���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1mol/L�����ƹ��������и�������������������ԭ����______��
A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ����
B������ʱ����������ƿ�̶��߽��ж���
C����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�����
D��ת����Һʱ��������������Һ��������ƿ����
E������ƿ������ˮϴ�Ӻ�δ���������������ˮ
F�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���
��1����ͼ�������Ľṹ��֪����Ϊ������ƿ�����������ܡ����Ƿ�Һ©��������100mL����ƿ��
�ʴ�Ϊ��������ƿ�������ܣ���Һ©����100mL����ƿ��
��2�����Һ©����100mL����ƿ��ʹ��ǰҪ����Ƿ�©ˮ��������ƿ�������ܲ���Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ���ۢܣ�
��3���������Ȼ�̼�е��ܽ�Ƚ���ˮ�д������Ȼ�̼��ˮ�������ܣ�������ȡ�ķ������룬���ſ��÷�Һ����������Һ��ֿ����ʷ����ˮ�еĵ�Ӧѡ��װ��II��
���Ȼ�̼�ӷ�����������ķ�������õ����Ȼ�̼������������Ȼ�̼��
������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ˮ�����ܣ�����ȡ���͵ⲻ��Ӧ����ѡ�ڢۣ�
�ʴ�Ϊ��II�����ڢۣ�
��4��a��û��480mL����ƿ��ѡ��500mL����ƿ��
���������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���ṩ��������֪����Ҫ�����У�500mL����ƿ����������
�ʴ�Ϊ��500mL����ƿ����������
b��ŨH2SO4�����ʵ���Ũ��c=
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��
����xmL��18.4mol/L=500mL��1mol/L����ã�x��27.2��
����Ũ��������Ϊ27.2mL����Ͳ���Խ�ӽ�����Ũ����������ԽС������ѡ��50mL��Ͳ��
�ʴ�Ϊ��27.2��B��
c��A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ��ȡŨ������������������ҺŨ��ƫ�ߣ���A���ϣ�
B������ʱ����������ƿ�̶��ߣ�Һ���ڿ̶������£�����������Һ�����С��������ҺŨ��ƫ�ߣ���B���ϣ�
C����Һ���������������ʣ�Ũ����ϡ�ͣ��ų��������ȣ��ܽ��δ�ָ���������ת�Ƶ�����ƿ�ж��ݣ�����������Һ�����С��������ҺŨ��ƫ�ߣ���C���ϣ�
D��ת����Һʱ��������������Һ��������ƿ���棬��������ƿ�е�������������ʵ�����С��������ҺŨ��ƫ�ͣ���D�����ϣ�
E�������Ҫ��ˮ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죬��E�����ϣ�
F��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ���F�����ϣ�
��ѡ��ABC��
�ʴ�Ϊ��������ƿ�������ܣ���Һ©����100mL����ƿ��
��2�����Һ©����100mL����ƿ��ʹ��ǰҪ����Ƿ�©ˮ��������ƿ�������ܲ���Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ���ۢܣ�
��3���������Ȼ�̼�е��ܽ�Ƚ���ˮ�д������Ȼ�̼��ˮ�������ܣ�������ȡ�ķ������룬���ſ��÷�Һ����������Һ��ֿ����ʷ����ˮ�еĵ�Ӧѡ��װ��II��
���Ȼ�̼�ӷ�����������ķ�������õ����Ȼ�̼������������Ȼ�̼��
������ȡ���е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���ȡ����ˮ�����ܣ�����ȡ���͵ⲻ��Ӧ����ѡ�ڢۣ�
�ʴ�Ϊ��II�����ڢۣ�
��4��a��û��480mL����ƿ��ѡ��500mL����ƿ��
���������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���ṩ��������֪����Ҫ�����У�500mL����ƿ����������
�ʴ�Ϊ��500mL����ƿ����������
b��ŨH2SO4�����ʵ���Ũ��c=
| 1000��1.84��98% |
| 98 |
����xmL��18.4mol/L=500mL��1mol/L����ã�x��27.2��
����Ũ��������Ϊ27.2mL����Ͳ���Խ�ӽ�����Ũ����������ԽС������ѡ��50mL��Ͳ��
�ʴ�Ϊ��27.2��B��
c��A������Ͳ��ȡŨ����ʱ�����ӿ̶���ȡŨ���ᣬ��ȡŨ������������������ҺŨ��ƫ�ߣ���A���ϣ�
B������ʱ����������ƿ�̶��ߣ�Һ���ڿ̶������£�����������Һ�����С��������ҺŨ��ƫ�ߣ���B���ϣ�
C����Һ���������������ʣ�Ũ����ϡ�ͣ��ų��������ȣ��ܽ��δ�ָ���������ת�Ƶ�����ƿ�ж��ݣ�����������Һ�����С��������ҺŨ��ƫ�ߣ���C���ϣ�
D��ת����Һʱ��������������Һ��������ƿ���棬��������ƿ�е�������������ʵ�����С��������ҺŨ��ƫ�ͣ���D�����ϣ�
E�������Ҫ��ˮ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죬��E�����ϣ�
F��ҡ�Ⱥ�Һ���½���һ������Һ����ƿ����ƿ��֮�䣬�ټ�����ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ���F�����ϣ�
��ѡ��ABC��

��ϰ��ϵ�д�
�����Ŀ