��Ŀ����
��2013?�ӳ�ģ�⣩��֪����Br2��Cl2һ��������������3Cl2+8NH3=N2+6NH4Cl
��þ������N2��ȼ�����ɻ���ɫ���壬��ˮ���ҷ�Ӧ�������ּ
ij��ѧѧϰС���������װ������ȡ������������ʵ�飮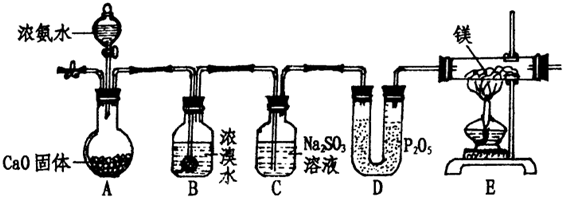
��1��ʵ�鲽�裺
�ٵ�ȼE���ƾ��ƣ�
�ڴ�A�з�Һ©��������
������װ�ã����װ�������ԣ�
��ͨ��������壬�ų�װ���ڿ�����
��װ�������ҩƷ��
�����˳����
��2��A������CaO��������
��3��д��B����Ӧ����ʽ
��4��C���������Ƶ������ǣ�
��5����˵����Ӧ������N2���ɵ�������
��6����װ�ò���֮����
��þ������N2��ȼ�����ɻ���ɫ���壬��ˮ���ҷ�Ӧ�������ּ
ij��ѧѧϰС���������װ������ȡ������������ʵ�飮

��1��ʵ�鲽�裺
�ٵ�ȼE���ƾ��ƣ�
�ڴ�A�з�Һ©��������
������װ�ã����װ�������ԣ�
��ͨ��������壬�ų�װ���ڿ�����
��װ�������ҩƷ��
�����˳����
�ۢݢܢ٢�
�ۢݢܢ٢�
��������ţ���2��A������CaO��������
CaO��ˮ�ҷų������ȣ������ڰ����ݳ�
CaO��ˮ�ҷų������ȣ������ڰ����ݳ�
����3��д��B����Ӧ����ʽ
3Br2+8NH3=N2+6NH4Br
3Br2+8NH3=N2+6NH4Br
����4��C���������Ƶ������ǣ�
��ȥ�����л���������Br2��NH3��Br2+SO32-+H2O=SO42-+2Br-+2H+��H++NH3=NH4+
��ȥ�����л���������Br2��NH3��Br2+SO32-+H2O=SO42-+2Br-+2H+��H++NH3=NH4+
�������ּ�������ӷ���ʽ����������5����˵����Ӧ������N2���ɵ�������
E���л���ɫ��������
E���л���ɫ��������
����6����װ�ò���֮����
������ֹ������ˮ�������룬ʹ�����ﲻ�����Ҳ���������Ⱦ����
������ֹ������ˮ�������룬ʹ�����ﲻ�����Ҳ���������Ⱦ����
����������1������ʵ������ȡ����������ʵ���Ŀ�Ľ��ʵ�鲽��������
��2��NH3+H2O?NH3?H2O?NH4++OH-��CaO��ˮ�ҷų������ȣ�ƽ�������ݳ������ƶ���
��3��Bװ����ȡ���������ݷ�Ӧ�ﰱ��������Br2��Cl2һ��������������Ϣ���
��4������������+4�۵�����л�ԭ�ԣ����������Ե����ʷ�����Ӧ��
��5��þ������N2��ȼ�����ɻ���ɫ���壬��Ӧ������N2���ɣ�E���л���ɫ�������ɣ�
��6��þ������N2��ȼ�����ɻ���ɫ���壬��ˮ���ҷ�Ӧ�������ּ��������ˮ������ͨ��E����װ�ý��룬��������Ⱦ�����壬����Ⱦ������
��2��NH3+H2O?NH3?H2O?NH4++OH-��CaO��ˮ�ҷų������ȣ�ƽ�������ݳ������ƶ���
��3��Bװ����ȡ���������ݷ�Ӧ�ﰱ��������Br2��Cl2һ��������������Ϣ���
��4������������+4�۵�����л�ԭ�ԣ����������Ե����ʷ�����Ӧ��
��5��þ������N2��ȼ�����ɻ���ɫ���壬��Ӧ������N2���ɣ�E���л���ɫ�������ɣ�
��6��þ������N2��ȼ�����ɻ���ɫ���壬��ˮ���ҷ�Ӧ�������ּ��������ˮ������ͨ��E����װ�ý��룬��������Ⱦ�����壬����Ⱦ������
����⣺��1��ͼʾװ�ã�A��ȡ������B��ȡ������C��ȥ�����е�Br2��NH3����þ������N2��ȼ�����ɻ���ɫ���壬��ˮ���ҷ�Ӧ�������ּ����D���ﵪ����EΪ������þ��Ӧ���ݴ˷�������һ��������װ�ã����װ�������ԣ��ڶ�����װ�������ҩƷ����������ͨ��������壬�ų�װ���ڿ�������������þ�ܷ�Ӧ������þ�͵�����Ӧ�����IJ��ٵ�ȼE���ƾ��ƣ����ڴ�A�з�Һ©��������ʼ��Ӧ��
�ʴ�Ϊ���ۢݢܢ٢ڣ�
��2����ˮ�д���ƽ��NH3+H2O?NH3?H2O?NH4++OH-��Ũ��ˮ�ӷ���CaO������ˮ��Ӧ�ų��������ȣ������¶����ߣ�ʹ�ð�����ˮ�е��ܽ�Ƚ�һ�����٣�ƽ�������ݳ������ƶ������������ʽ�ݳ����Ƶð�����
�ʴ�Ϊ��CaO��ˮ�ҷų������ȣ������ڰ����ݳ���
��3��Aװ����ȡ������ͨ��Bװ�ã�Bװ��װ��Ũ��ˮ��Br2��Cl2һ��������������3Cl2+8NH3=N2+6NH4Cl�����Ȼ����弴�ɵõ�B����Ӧ����ʽ��3Br2+8NH3=N2+6NH4Br��
�ʴ�Ϊ��3Br2+8NH3=N2+6NH4Br��
��4��Cװ����װ���������ƣ�����������+4�۵�����л�ԭ���ܺ�Bװ�����ų��Ķ�����巴Ӧ��Br2+SO32-+H2O=SO42-+2Br-+2H+�����ɵ���ɺͰ�����Ӧ��H++NH3=NH4+������Cװ�õ�����Ϊ��ȥ�����е�Br2��NH3��
�ʴ�Ϊ����ȥ�����л���������Br2��NH3��Br2+SO32-+H2O=SO42-+2Br-+2H+��H++NH3=NH4+��
��5��EΪ������þ��Ӧ��װ�ã�ֻҪ�е�������3Mg+N2
Mg3N2��Mg3N2Ϊ��ɫ���壬������˵����Ӧ������N2���ɵ�������E���л���ɫ�������ɣ�
�ʴ�Ϊ��E���л���ɫ�������ɣ�
��6����װ�������ð���������ص�ʵ�飬�����Ǵ�����Ⱦ���β������װ�ã�����þ��ˮ���ҷ�Ӧ�������ּ��Eװ��������������ֹ�����е�ˮ�������룬
�ʴ�Ϊ��������ֹ������ˮ�������룬ʹ�����ﲻ�����Ҳ���������Ⱦ������
�ʴ�Ϊ���ۢݢܢ٢ڣ�
��2����ˮ�д���ƽ��NH3+H2O?NH3?H2O?NH4++OH-��Ũ��ˮ�ӷ���CaO������ˮ��Ӧ�ų��������ȣ������¶����ߣ�ʹ�ð�����ˮ�е��ܽ�Ƚ�һ�����٣�ƽ�������ݳ������ƶ������������ʽ�ݳ����Ƶð�����
�ʴ�Ϊ��CaO��ˮ�ҷų������ȣ������ڰ����ݳ���
��3��Aװ����ȡ������ͨ��Bװ�ã�Bװ��װ��Ũ��ˮ��Br2��Cl2һ��������������3Cl2+8NH3=N2+6NH4Cl�����Ȼ����弴�ɵõ�B����Ӧ����ʽ��3Br2+8NH3=N2+6NH4Br��
�ʴ�Ϊ��3Br2+8NH3=N2+6NH4Br��
��4��Cװ����װ���������ƣ�����������+4�۵�����л�ԭ���ܺ�Bװ�����ų��Ķ�����巴Ӧ��Br2+SO32-+H2O=SO42-+2Br-+2H+�����ɵ���ɺͰ�����Ӧ��H++NH3=NH4+������Cװ�õ�����Ϊ��ȥ�����е�Br2��NH3��
�ʴ�Ϊ����ȥ�����л���������Br2��NH3��Br2+SO32-+H2O=SO42-+2Br-+2H+��H++NH3=NH4+��
��5��EΪ������þ��Ӧ��װ�ã�ֻҪ�е�������3Mg+N2
| ||
�ʴ�Ϊ��E���л���ɫ�������ɣ�
��6����װ�������ð���������ص�ʵ�飬�����Ǵ�����Ⱦ���β������װ�ã�����þ��ˮ���ҷ�Ӧ�������ּ��Eװ��������������ֹ�����е�ˮ�������룬
�ʴ�Ϊ��������ֹ������ˮ�������룬ʹ�����ﲻ�����Ҳ���������Ⱦ������
���������⿼�鰱��������þ���Ʊ�������ʱҪ��ץ�����Ϣ��ϰ���������þ�����ʣ��������ʵ��װ��ͼ���ص��ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
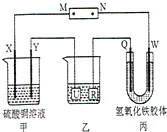 ��2013?�ӳ�ģ�⣩��ͼ��ʾ��X��Y��Q��W ���Ƕ��Ե缫������Դ��ͨ��W��������ɫ�������˵���д�����ǣ�������
��2013?�ӳ�ģ�⣩��ͼ��ʾ��X��Y��Q��W ���Ƕ��Ե缫������Դ��ͨ��W��������ɫ�������˵���д�����ǣ������� ��2013?�ӳ�ģ�⣩�����������A��B��Ϊͬ���칹�壬����C��H��O����Ԫ�أ�
��2013?�ӳ�ģ�⣩�����������A��B��Ϊͬ���칹�壬����C��H��O����Ԫ�أ�


 ��ͬ���칹��ܶ࣬д�����к��������������������ͬ���칹��Ľṹ��ʽ��
��ͬ���칹��ܶ࣬д�����к��������������������ͬ���칹��Ľṹ��ʽ��