��Ŀ����
�����10�֣�
2005���ŵ������ѧ���������ϩ�����ֽⷴӦ�о���������ͻ������3λ��ѧ�ҡ�ϩ�����ֽⷴӦʵ������һ��������ϩ����̼̼˫�������ŵĻ�λ��
�磺2CH2=CHCH2CH3 CH2��CH2+CH3CH2CH=CHCH2CH3��
CH2��CH2+CH3CH2CH=CHCH2CH3��
����֪������ȩ������һ�������¿����ȷ����ӳɷ�Ӧ��������ȥ��Ӧ��

�ֽ��Ա�ϩΪ�л�ԭ�ϣ��������з�Ӧ���Էֱ�ϳ���Ҫ�Ļ���ԭ��F��K����F��KΪԭ�Ͽɺϳ�һ����״�߷��ӻ�����M���仯ѧ���Ϊ(C12H20O4)n��

�ش��������⣺
43����Ӧ�ٵķ�Ӧ������_______________��
44����Ӧ�ޡ�������һ��Ӧ����HCl�ӳɣ��÷�Ӧ��_____���Ӧ��ţ��������һ����Ӧ��Ŀ����_____________________________________________________��
45������M�Ľṹ��ʽΪ��______________________________________��
46��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ࣺ_____________________________________________________________��
��Ӧ�⣺_____________________________________________________________��
2005���ŵ������ѧ���������ϩ�����ֽⷴӦ�о���������ͻ������3λ��ѧ�ҡ�ϩ�����ֽⷴӦʵ������һ��������ϩ����̼̼˫�������ŵĻ�λ��
�磺2CH2=CHCH2CH3
 CH2��CH2+CH3CH2CH=CHCH2CH3��
CH2��CH2+CH3CH2CH=CHCH2CH3������֪������ȩ������һ�������¿����ȷ����ӳɷ�Ӧ��������ȥ��Ӧ��

�ֽ��Ա�ϩΪ�л�ԭ�ϣ��������з�Ӧ���Էֱ�ϳ���Ҫ�Ļ���ԭ��F��K����F��KΪԭ�Ͽɺϳ�һ����״�߷��ӻ�����M���仯ѧ���Ϊ(C12H20O4)n��

�ش��������⣺
43����Ӧ�ٵķ�Ӧ������_______________��
44����Ӧ�ޡ�������һ��Ӧ����HCl�ӳɣ��÷�Ӧ��_____���Ӧ��ţ��������һ����Ӧ��Ŀ����_____________________________________________________��
45������M�Ľṹ��ʽΪ��______________________________________��
46��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ࣺ_____________________________________________________________��
��Ӧ�⣺_____________________________________________________________��
43. �ӳɷ�Ӧ ��1�֣�
44. �ޣ�1�֣� ����B(��G)�����е�C��C����������2�֣�
45. ��2�֣�
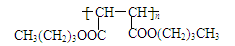
46. HOOCCH2CHClCOOH+3NaOH
 NaOOCCH��CHCOONa+NaCl+3H2O ��2�֣�
NaOOCCH��CHCOONa+NaCl+3H2O ��2�֣�2CH3(CH2)3OH+HOOCCH��CHCOOH
 CH3(CH2)3OOCCH��CHCOO(CH2)3CH3+2H2O ��2�֣�
CH3(CH2)3OOCCH��CHCOO(CH2)3CH3+2H2O ��2�֣�������������������жϵڶ�����Ӧ������Ϣ�ṩ�Ļ��Ž�����Ӧ������Ϊ��ϩ��ClCH2CH=CHCH2Cl��3CH=CHCHO��FΪCH3CH2CH2CH2OH��Bˮ��ΪHOCH2CH=CHCH2OH��ͨ���ӳ�HCl����̼̼˫�����������ǻ�Ϊ�Ȼ�����ȥ�γ�̼̼˫������F������Ӧ�õ�L����Ӿ۵õ�M��

��ϰ��ϵ�д�
�����Ŀ
 ������Ϊ2,7,7-����-3-�һ�����
������Ϊ2,7,7-����-3-�һ����� ��ʹBr2/CCl4��Һ��ɫ��˵���÷����д��ڶ�����̼̼������̼̼˫��
��ʹBr2/CCl4��Һ��ɫ��˵���÷����д��ڶ�����̼̼������̼̼˫�� �������������14��̼ԭ�ӹ�ƽ��
�������������14��̼ԭ�ӹ�ƽ�� �ĵ�����CH3-C��C-CH3��CH2=CH-CN
�ĵ�����CH3-C��C-CH3��CH2=CH-CN

 �����ʲ����еĻ�ѧ�����ǣ� ��
�����ʲ����еĻ�ѧ�����ǣ� ��
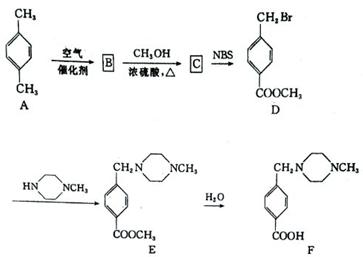
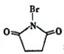 ����ӦC��D�Ļ�ѧ����ʽΪ��������������������������������
����ӦC��D�Ļ�ѧ����ʽΪ��������������������������������