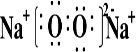��Ŀ����
| �� ���� |
IA | 0 | ||||||
| 1 | H | ��A | ��A | ��A | ��A | ��A | ��A | He |
| 2 | Li | Be | B | C | N | O | F | Ne |
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
��1�����ƶ����Ǹ���ʲôԪ�أ�д�����ǵ�Ԫ�ط��ţ�
A______��B______��C��______��D______��E______��
��2��д��A�ֱ���B��C��D���γɻ�����Ļ�ѧʽ��______��______��______
��3��д��A��C��D�γɵĻ�������A��D��E�γɵĻ���������Ӧ�Ļ�ѧ����ʽ��______��
1��18��Ԫ���У���A��B��C��D��E����Ԫ�أ�����ԭ�ӵĺ˵�������ε����Ҿ�С��18��Aԭ�Ӻ��ڽ���1�����ӣ�AΪHԪ�أ�Bԭ�ӵ�����������Ϊ���ڲ��������2����B��2�����Ӳ㣬����������Ϊ4����BΪCԪ�أ�Dԭ�����������Ӳ㣬�����������Ǵ�����������3��������������Ϊ6����DΪOԪ�أ�EԪ�ش��ڵ������ڣ�EԪ�ص�����������������Ӳ���������֮һ��������������Ϊ2����EΪMgԪ�أ�Aԭ����Bԭ�ӵ�����������֮����Cԭ�ӵ�������������ȣ���C������������Ϊ1+4=5��ԭ������С��OԪ�أ���CΪNԪ�أ���
��1�����������ӿ�֪��AΪHԪ�أ�BΪCԪ�أ�CΪNԪ�أ�DΪOԪ�أ�EΪMgԪ�أ�
�ʴ�Ϊ��H��C��N��O��Mg��
��2��H�ֱ���C��N��O���γɻ�����Ļ�ѧʽΪCH4��NH3��H2O�ȣ�
�ʴ�Ϊ��CH4��NH3��H2O��
��3��A��C��D�γɵĻ�������A��D��E�γɵĻ���������Ӧ����A��C��D�γɵĻ�����ΪHNO3��A��D��E�γɵĻ�����
ΪMg��OH��2�����߷�����Ӧ�ķ���ʽΪ��Mg��OH��2+2HNO3=Mg��NO3��2+2H2O��
�ʴ�Ϊ��Mg��OH��2+2HNO3=Mg��NO3��2+2H2O��
��1�����������ӿ�֪��AΪHԪ�أ�BΪCԪ�أ�CΪNԪ�أ�DΪOԪ�أ�EΪMgԪ�أ�
�ʴ�Ϊ��H��C��N��O��Mg��
��2��H�ֱ���C��N��O���γɻ�����Ļ�ѧʽΪCH4��NH3��H2O�ȣ�
�ʴ�Ϊ��CH4��NH3��H2O��
��3��A��C��D�γɵĻ�������A��D��E�γɵĻ���������Ӧ����A��C��D�γɵĻ�����ΪHNO3��A��D��E�γɵĻ�����
ΪMg��OH��2�����߷�����Ӧ�ķ���ʽΪ��Mg��OH��2+2HNO3=Mg��NO3��2+2H2O��
�ʴ�Ϊ��Mg��OH��2+2HNO3=Mg��NO3��2+2H2O��

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ
�±�ΪԪ�����ڱ���һ���֣��й�˵����ȷ���ǣ�������
| �� ���� |
IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
| A���ܡ��ݡ��ޡ��ߡ��ٵ�ԭ�Ӱ뾶���μ�С |
| B���ݡ��ޡ��������������Ӧ��ˮ���ﲻ�����Ӧ |
| C���ߡ��ڡ��ۡ��ܵ��⻯����ȶ���������ǿ |
| D���ڡ��۵���̬�⻯����������ǵ�����������Ӧ��ˮ���ﷴӦ |