��Ŀ����
��ҵ���ô�����ͭ(������FeO)Ϊԭ����ȡ�Ȼ�ͭ����(CuCl2��2H2O)�������������£�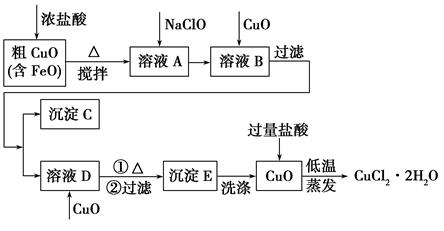
| ���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH | ��9.6 | ��6.4 | 3��4 |
(2)����C�Ļ�ѧʽΪ________��
(3)ʵ������μ������E��ϴ�Ӹɾ���________��
(4)����������Ŀ����______________________________________��
(5)���������μ�����CuO��������һ��������ҺB�м��������CuO����������________________________________________________��
(1)2Fe2����ClO����2H��=2Fe3����Cl����H2O
(2)Fe(OH)3
(3)ȡ���һ��ϴ��Һ�������Թ��У�����AgNO3��Һ���ް�ɫ�������ɣ�˵����ϴ�ɾ�
(4)����Cu2��ˮ�⣬��ֹ�����нᾧˮʧȥ(���һ�㼴��)
(5)һ���Լ��������CuO������Fe3����Cu2��ͬʱ���ɳ���
����

������[KAl(SO4)2��12H2O]�Ʊ�Al��K2SO4��H2SO4���������£�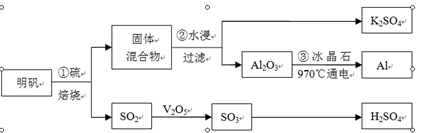
�������յĻ�ѧ����ʽΪ��4KAl(SO4)2��12H2O+3S��2K2SO4 +2Al2O3+9SO2��+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У��������� ��
��2��������У�Ϊ��߽����ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
| A������������� | B�������¶� | C�����Ͻ��� | D�����̽���ʱ�� |
��4������۵��Ļ�ѧ����ʽ�� �����صĵ缫����̼�ز������ɣ��������У�����������Ҫ���ڸ�����ԭ���ǣ� ��
��5����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2����õ�ص������缫��Ӧʽ�� ��
��6������a��������Ħ������Ϊb g/mol������SO2��ת����Ϊ96%����������������Ϊ98����H2SO4����Ϊ ��(�г��������ʽ)��
�ճ�������ʹ�õ����Ͻ��е��������ڵ������������ҵ�ϵ��������Ҫ���䴿�Ȳ��õ���98.2%������Ȼ�����������������Ϊ50%��70%��������ҪΪSiO2��Fe2O3��CaO��MgO��Na2O�ȡ���ҵ���������Ĺ�������ʾ��ͼ���£�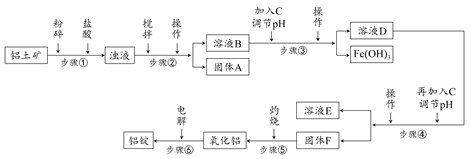
�� һЩ�������������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Mg(OH)2 |
| ��ʼ����pH(���ӳ�ʼŨ��0.01 mol/L) | 4 | 2.3 | 10.4 |
| ��ȫ����pH(����Ũ��<10��5 mol/L) | 5.2 | 4.1 | 12.4 |
(1)��������ʱ��������������Ӧ�����ӷ���ʽΪ________________��
(2)ʵ���ҽ��в���ڢۢܵIJ�������Ϊ________��
(3)����A�Ļ�ѧʽΪ________������C�Ļ�ѧʽΪ________����Һ�е�Na����Ca2����Mg2�����ڲ���________�з����ȥ�ġ�
(4)����۵���pH����ֵ��ΧΪ________������ܵ���pH����ֵ��ΧΪ________��
(5)�����Ӧ�Ļ�ѧ����ʽΪ________��
ij�о�С�齫һ����������·�徭Ũ�����ϡ���ᴦ����õ�һ�����Һ,���к���Cu2+��Fe2+��Fe3+��Al3+�Ƚ�������,��������������������Էֱ���ȡCuSO4��5H2O�����AlCl3
��Һ: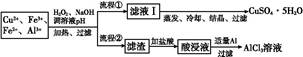
��֪:��ؽ������ӿ�ʼ��������ȫ����ʱ��pH��ΧΪ:
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
(1)����H2O2���������� ,��ʹ��ȡ��CuSO4��5H2O�����Ϊ����,pH����Ӧ��������������
(2)д��H2O2��Fe2+��Ӧ�����ӷ���ʽ: ��
(3)���̢��м�������Al����������� ��������
(4)��������ѧ�Ļ�ѧ֪ʶ,��AlCl3��Һ(������������ѧ�Լ�)�ܷ��Ƶ���ˮAlCl3��������(��ܡ����ܡ�),ԭ������ ��
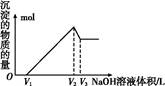
(5)ȡ���ΪV(L)�����Һ,�����еμ�a mol��L-1��NaOH��Һ,���ɳ��������ʵ��������ӵ�NaOH��Һ�����(L)��ϵ��ͼ������V1��V2��V3��ʾ��ȡ�����Һ��n(Fe3+)��n(Al3+)=�� ��

 2K2S+K2SO3+3H2O���÷�Ӧ���������뻹ԭ������֮��Ϊ___________��
2K2S+K2SO3+3H2O���÷�Ӧ���������뻹ԭ������֮��Ϊ___________��