��Ŀ����
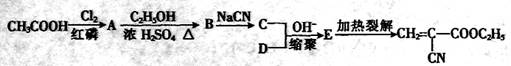
(15��)502��������˲�ɽ�����Ч�ɷ���a��������ϩ������������̻��ٶȿ죬ʹ�÷��㣬ճ����ǿ���㷺Ӧ���ڵ�����ҽ�Ƶ���ҵ����������·�ߺϳɣ�
��֪
��D������װ��ʱ�������Ⱦ���塣
�ش��������⣺
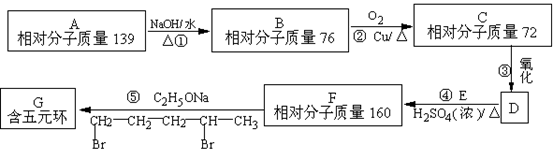
(1) B�Ľṹ��ʽ ��______��������(ϵͳ��������______��
(2) E�ĵ����к��еĹ�������_______(д���ƣ���
(3) A��������NaOHˮ��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��__________________
(4) д��C��D�ڼ��������·�Ӧ���ɾۺ���E�Ļ�ѧ����ʽ__________________
(5) X��C��ͬϵ�����һ��̼ԭ�ӣ���X���п��ܵĽṹ��ʽ��____________

��֪

��D������װ��ʱ�������Ⱦ���塣
�ش��������⣺
(1) B�Ľṹ��ʽ ��______��������(ϵͳ��������______��
(2) E�ĵ����к��еĹ�������_______(д���ƣ���
(3) A��������NaOHˮ��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��__________________
(4) д��C��D�ڼ��������·�Ӧ���ɾۺ���E�Ļ�ѧ����ʽ__________________
(5) X��C��ͬϵ�����һ��̼ԭ�ӣ���X���п��ܵĽṹ��ʽ��____________
����15�֣�
��1��CH2ClCOOC2H5��1�֣� 2-������������1�֣�
��2��̼̼˫���������������3�֣�
��3��CH2ClCOOH+2NaOHCH2��OH��COONa+NaCl+H2O��3�֣�
��4��nCOOC2H5+nHCHOnH2O+CH2��CN?��3�֣�
��5��HCOOCH3��HCOOCH2��COOCH3��CH3COO��4�֣�
��1��CH2ClCOOC2H5��1�֣� 2-������������1�֣�
��2��̼̼˫���������������3�֣�
��3��CH2ClCOOH+2NaOHCH2��OH��COONa+NaCl+H2O��3�֣�
��4��nCOOC2H5+nHCHOnH2O+CH2��CN?��3�֣�
��5��HCOOCH3��HCOOCH2��COOCH3��CH3COO��4�֣�
��

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�
�����Ŀ


 ��������գ�
��������գ�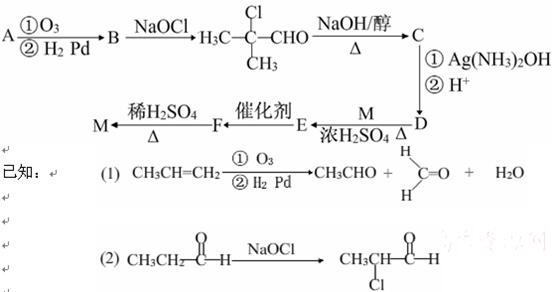

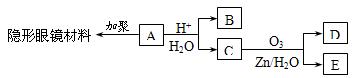
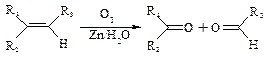


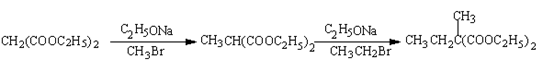
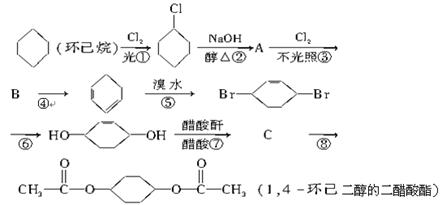
 �л���A��һ�������¿��Է�������ͼ��ʾ��ת������������
�л���A��һ�������¿��Է�������ͼ��ʾ��ת������������ ��ˮ��ʡ�ԣ�������������ʵĽṹ������Ϊ��A����������������Ʒ�Ӧ����������B����̼ԭ������A��ͬ����ֻ����һ�ֹ���
��ˮ��ʡ�ԣ�������������ʵĽṹ������Ϊ��A����������������Ʒ�Ӧ����������B����̼ԭ������A��ͬ����ֻ����һ�ֹ��� �ţ�C�ܷ���������Ӧ��1molD������̼��������Һ��Ӧ�ɲ���������̼2mol��
�ţ�C�ܷ���������Ӧ��1molD������̼��������Һ��Ӧ�ɲ���������̼2mol��