��Ŀ����
����Ŀ��ijУ��ѧ��ȤС���ͬѧ����ɿα��Ҵ�����ȩ��ʵ��Ľ���ʵ�鷽������ͼ��������˳���������ʵ�������
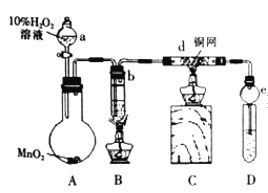
�����Ӻ�����������װ�õ������ԣ�
����ȷ�����Լ���
�۵�ȼCװ���еľƾ��ƣ�
����Բ����ƿ�еμ�10������������Һ��
�ݵ�ȼBװ���еľƾ��Ƽ�����ˮ�Ҵ������ڲ��������ȡ�
���������������ݻش��������⣺
��1��װ��e��������______________��
��2��д��d�з�����Ӧ�Ļ�ѧ����ʽ__________________________��
��3��Ϊ����D���ռ��IJ������Ƿ�����ȩ����ͬѧ����������Һ���飬��д����ѧ��Ӧ����ʽ_______________________��
��4������ȤС���ͬѧ������ȩ����������Ӧ�����ʵ������������̽��������ʵ���������±�����
ʵ����� ʵ����� | ������Һ��S/mL | ��ȩ����/�� | �¶�/�� | ��Ӧ���Һ��pHֵ | ����������ʱ��/min |
1 | 1 | 3 | 65 | 11 | 5 |
2 | 1 | 3 | 45 | 11 | 6.5 |
3 | 1 | 5 | 65 | 11 | 4 |
��ʵ��1��ʵ��2��̽����ʵ��Ŀ����_________________________��
�ڵ�������Һ����ΪlmL����ȩ����Ϊ3�Σ��¶�Ϊ55�棬��Ӧ���ҺpHΪ11ʱ������������ʱ��Ϊ_________min�����Χ��
��5�����Թ����ռ�����Һ������ɫʯ����Һ���飬��Һ�Ժ�ɫ��˵��Һ���л�����___________�����������ƣ���Ҫ��ȥ�����ʣ������ڻ��Һ�м���_________����д����ѡ���е���ĸ����Ȼ����ͨ��___________����������ƣ����ɳ�ȥ��
A.����NaCl��Һ B.C2H5OH C.NaHCO3��Һ D.CCl4
���𰸡� �����ռ���Ʒ������ֹ���� 2CH3CH2OH��O2![]() 2CH3CHO��2H2O��Cu+O2
2CH3CHO��2H2O��Cu+O2![]() 2CuO��CH3CH2OH+CuO��CH3CHO+Cu+H2OҲ��ȷ�� CH3CHO+2Ag��NH3��2OH
2CuO��CH3CH2OH+CuO��CH3CHO+Cu+H2OҲ��ȷ�� CH3CHO+2Ag��NH3��2OH![]() CH3COONH4+2Ag��+3NH3+H2O �¶ȶԷ�Ӧ���ʵ�Ӱ�� 5��6.5 ���� C ����
CH3COONH4+2Ag��+3NH3+H2O �¶ȶԷ�Ӧ���ʵ�Ӱ�� 5��6.5 ���� C ����
�����������������������Ҫ��������Ҵ�����ȩʵ������ۡ�
��1��װ��e�������������ռ���Ʒ������ֹ������
��2��d���Ҵ�������Ϊ��ȩ��������Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O ��
2CH3CHO��2H2O ��
��3��������Ӧ�Ļ�ѧ����ʽΪCH3CHO+2Ag��NH3��2OH![]() CH3COONH4+2Ag��+3NH3+H2O��
CH3COONH4+2Ag��+3NH3+H2O��
��4����ʵ��1��ʵ��2���¶Ȳ�ͬ��̽����ʵ��Ŀ�����¶ȶԷ�Ӧ���ʵ�Ӱ�졣
����������Һ����ΪlmL����ȩ����Ϊ3�Σ��¶�Ϊ55������Ӧ���ҺpHΪ11ʱ������������ʱ�����45����65��ʱ��ʱ��֮��Ϊ5��6.5min��
��5�����Թ����ռ�����Һ������ɫʯ����Һ���飬��Һ�Ժ�ɫ��˵��Һ���л�������ȩ�������������ᣬҪ��ȥ�����ʣ�����CH3COOH+ NaHCO3![]() CH3COONa+H2O+CO2���������ڻ��Һ�м���C����ȩ����ˮ������ʹ�÷�Һ����������ȩ��ˮ�е�IJ��Ȼ����ͨ�����ɳ�ȥ��
CH3COONa+H2O+CO2���������ڻ��Һ�м���C����ȩ����ˮ������ʹ�÷�Һ����������ȩ��ˮ�е�IJ��Ȼ����ͨ�����ɳ�ȥ��

����Ŀ��ijʵ��С���Դ�����������Fe��Cr���ʣ�Ϊԭ���Ʊ�Ni(NH3)6Cl2�����ⶨ�����ֵĺ������Ʊ�����ʾ��ͼ���£�

��֪�����������������������������pH(��ʼ������pH������Ũ��Ϊ0.1 mol��L-1����)���±���ʾ��
���� | Fe3+ | Cr3+ | Ni2+ |
��ʼ����pH | 1.5 | 4.3 | 6.9 |
��ȫ����pH | 2.8 | 5.6 | 8.9 |
��Ni(OH)2Ϊ��ɫ�����Ni(NH3)6(NO3)2��Ni(NH3)6Cl2��Ϊ������ˮ������ɫ���壬ˮ��Һ���Լ��ԡ�
�ش��������⣺
��1��ʵ����Ҫ����3.0mol��L-1ϡ����250mL����Ҫ�IJ����������ձ�����Ͳ������������ͷ�ιܺ�_________________��
��2������(a)��Ni��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ________________��
��3������(b)���ȼ����� ��X������Һ��pHԼΪ6�����˺��ټ�������X����pH�Եõ���ɫ������
������pHԼΪ6��ԭ����_______________________________________________��
���Լ�X������__________________(����)��
A. H2SO4 B. Ni(OH)2 C. NaOH D. Fe2O3 E. NiO
��4��NH3�����IJⶨ
i. �õ�����ƽ����mg��Ʒ����ƿ�У���25mLˮ�ܽ�����3.00mL 6 mol/L���ᣬ�Լ�����ָʾ�����ζ����յ�����0.500 0 mol��L-1NaOH����ҺV1mL��
ii. �հ����飺��������Ʒ�ظ�ʵ��i������NaOH����ҺV2mL��
NH3����������Ϊ____________��(��V1 ,V2��ʾ)
�������������Ļ����ϣ����д�ʩ�ܽ�һ����߲ⶨȷ�ȵ���____________�����ţ���
A. �ʵ���߳�����Ʒ������ B. ��H2SO4��Һ�������
C. �÷�̪������� D. ����ƽ��ʵ��
��5��Ϊ�ⶨCl���ĺ������벹����������ʵ�鷽����
����mg��Ʒ����ƿ�У���25mLˮ�ܽ⣬________________������2��3��K2CrO4��Һ��ָʾ��������֪Ũ�ȵ�AgNO3����Һ�ζ����յ㣬��¼�������ظ�����2��3�Ρ�

