��Ŀ����
13�� ������ķ���ʽΪC6H5COOH�����ӽṹΪ
������ķ���ʽΪC6H5COOH�����ӽṹΪ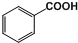 ���DZ����ϵ�һ���ⱻ�Ȼ���-COOH��ȡ���γɵĻ�����仯ѧ�������������ƣ����������ԣ������Ժ��Ҵ�����������Ӧ���ɱ�����������
���DZ����ϵ�һ���ⱻ�Ȼ���-COOH��ȡ���γɵĻ�����仯ѧ�������������ƣ����������ԣ������Ժ��Ҵ�����������Ӧ���ɱ�������������֪�����������ķе�Ϊ213�棬��ˮ���Ҵ��ͻ������ܹ���7.0%��17.0%��76.0%�ı�����62.1��ʱ��Ϊ�����ݳ��������������Ϣ��װ��ͼ�ش�����ʵ�����Ʊ��������������й����⣺
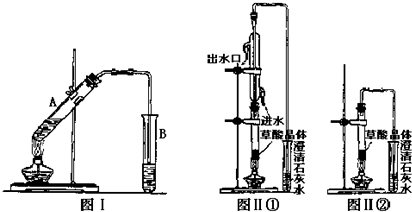
��1����������Բ����ƿ�м��뱽���ᡢŨ���ᡢ�������Ҵ�����ʯ����������ƿ�м��뻷���飬װ�Ϸ�ˮ���Ļ��������ܣ�д���Ʊ������������ķ���ʽ��
 +CH3CH2OH$?_{��}^{Ũ����}$
+CH3CH2OH$?_{��}^{Ũ����}$ +H2O��ʵ����ʹ�÷�ˮ����Ŀ���Ƿ�������ɵ�ˮ��������ƽ�������������������ķ����ƶ�����
+H2O��ʵ����ʹ�÷�ˮ����Ŀ���Ƿ�������ɵ�ˮ��������ƽ�������������������ķ����ƶ�������2���������Ȼ���������ˮ���²�Һ�岻�����ֹ࣬ͣ���ȣ��ų���ˮ����Һ�壬��ˮ����Һ���������Ҫ�ɷ��ǣ��¶ȼ�ˮ��ˮ���Ҵ��������飮
��3����Բ����ƿ�еIJ�Һ����ʢ����ˮ����ƿ�У��ñ���̼������Һ�к��������ԣ���Һ���ֳ��л���ֲ�Ʒ��ˮ�������ѣ�����Ϊ�����л��ܼ���ˮ���Խϲ�е�Ϊ34.5�棩��ȡ����ʵ��������ƣ������Ѳ���ֲ�Ʒ�ϲ����ô�ˮϴ�л������Σ������Ѳ���ˮ�����־������Ѳ���Ͽڵ���һ���������ƿ��
��4����������������С����ˮ�Ȼ��Ƹ������ҡ����ƿ�������Ѳ�����������Ѳ������һ�������Բ����ƿ������������ʵ��������ƣ������������ѣ�������������������
���� ��1����ѧ��Ӧ����ʽΪ�� +CH3CH2OH$?_{��}^{Ũ����}$
+CH3CH2OH$?_{��}^{Ũ����}$ +H2O����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
+H2O����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
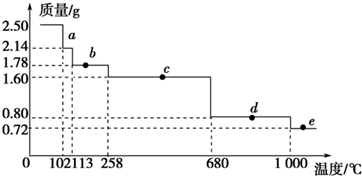
��2���е�͵������ȱ�����õ�����Ԫ�������л����麬������Ҵ��ǹ������ʣ������ٴ�����õ��Ҵ��ͻ����飻�¶ȵ��ڱ����������ķе�ʱ�����������������������
��3�������������к���������������̼������Һ�е��ܽ�Ƚ�С�����뻥�����ܵ�Һ����÷�Һ�ķ������룬����Ϊ�����л��ܼ���ˮ���Խϲ������ȡ���ɵ������������
��4�������ӷ���˵�����ѷе�ϵͣ������������е�������ѣ��������ʷе㲻ͬ��ͨ�������¶�����õ����Ѻ�����
��� �⣺��1�����������Ϣ֪����������Ҵ���Ӧ���ɱ�����������ˮ����ѧ��Ӧ����ʽΪ�� +CH3CH2OH$?_{��}^{Ũ����}$
+CH3CH2OH$?_{��}^{Ũ����}$ +H2O��
+H2O��
�ʴ�Ϊ�� +CH3CH2OH$?_{��}^{Ũ����}$
+CH3CH2OH$?_{��}^{Ũ����}$ +H2O��ˮ-�Ҵ�-���������γ���Ԫ���������ˮ�����٣�������������٣�����ƽ��������Ӧ�����ƶ�����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
+H2O��ˮ-�Ҵ�-���������γ���Ԫ���������ˮ�����٣�������������٣�����ƽ��������Ӧ�����ƶ�����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
�ʴ�Ϊ�� +CH3CH2OH$?_{��}^{Ũ����}$
+CH3CH2OH$?_{��}^{Ũ����}$ +H2O����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
+H2O����������ɵ�ˮ��������ƽ�������������������ķ����ƶ���
��2���е�͵������ȱ�����õ�����Ԫ��������۵�ϵͣ�������Ԫ�������ȱ�����õ������Ը�Һ���������Ҫ�ɷ���ˮ���Ҵ��������飻
�ʴ�Ϊ��ˮ���Ҵ��������飻
��3��̼����Ϊǿ�������Σ�̼������Һ�ʼ��ԣ��ܺ��ᷴӦ����ȥ�ᣬ̼������Һ�ܽ��������ܽ�ȣ�����̼������Һ�������ǣ���ȥ��Ʒ�е��������ʡ����ͱ������������ܽ�ȣ������ñ���̼������Һ�к��������ԣ���ȥ���ᡢ�Ҵ������뻥�����ܵ�Һ����÷�Һ�ķ������룬��Ҫ������Ϊ��Һ©������Һ���ֳ��л���ֲ�Ʒ��ˮ����������ȡ���룬
�ʴ�Ϊ������̼���ƣ���ȡ��
��4�������ӷ�˵�����ѵķе�ϵͣ������������е�ϸߣ�����ʱ���е�͵����������е�ߵĺ����������Խ��Ѳ���뵽�����������ƿ�н���������������������ѡ�������������������
�ʴ�Ϊ���������ѣ�
���� ���⿼���������Ʊ�����ȷʵ��ԭ���ǽⱾ��ؼ�������ʵ��ԭ�������ʵ�������������𣬺ܶ�ѧ�����л���ѧʵ��֪ʶ�˽���٣����ʱ�׳��ִ���Ҫ�������ս̲Ļ���֪ʶ��������û���֪ʶ�����������Ŀ�Ѷ��еȣ�

| A�� | ��Һ�и����ӵ�Ũ�ȶ���С | B�� | c��Cl-��/c��NH4+����С | ||
| C�� | c��H+��/c��NH4+������ | D�� | c��H+��•c��OH-����С |
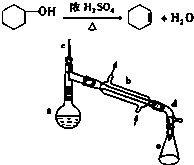 ����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɼ�ϩ�ķ�Ӧ��ʵ��װ����ͼ��
����ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɼ�ϩ�ķ�Ӧ��ʵ��װ����ͼ�������õ����й��������£�
| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | �ܽ��� | |
| ������ | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
�����ᴿ��
��Ӧ�ֲ��ﵹ���Һ©���зֱ�������5%̼������Һ��ˮϴ�ӣ�����������ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�����ͨ������õ���������ϩl0g��
�ش��������⣺
��1��װ��a��������������ƿ��
��2���������Ƭ�������Ƿ�ֹ���У�
��3����ʵ���������ײ����ĸ�����Ľṹ��ʽΪ
 ��
����4����Һ©����ʹ��ǰ���©���ڱ�ʵ���������У�����Ӧ�ôӷ�Һ©�����Ͽڵ�������Ͽڵ��������¿ڷų�������
��5�������ᴿ�����м�����ˮ�Ȼ��Ƶ�Ŀ��������ˮ��
��6����ʵ�����õ��Ļ���ϩ������C������ȷ�𰸱�ţ���
A��41% B��50% C��61% D��70%
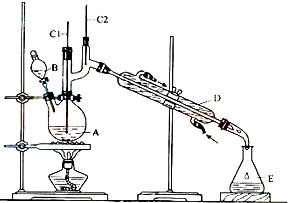
CH3CH2CH2CH2OH$��_{H_{2}SO_{4}����}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��6.0g Na2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90-95�棬��E���ռ�90�����µ���֣�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75-77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ��������Ž���
��2�������ʯ�������Ƿ�ֹ���У�
��3������װ��ͼ�У�B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
a����ʪb������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���²㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90-95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��7����ʵ���У�����ȩ�IJ���Ϊ51.4%��
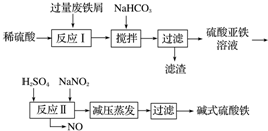
��֪������������������������ʽ����ʱ��Һ��pH�����ʾ��
| ������ | Fe��OH��3 | Fe��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
��1����������NaHCO3��Ŀ���ǵ���pH��ʹ��Һ�е�Al��OH��3�������ù����С����衱��������ʹ��Ӧ���ֽӴ���Ӧ��
��2����ʵ�������У���Ӧ���г�ͬʱͨ��O2�Լ���NaNO2��������O2��NaNO2�ڷ�Ӧ�о����������������뷴Ӧ��O2��11.2L����״���������൱�ڽ�ԼNaNO2�����ʵ���Ϊ2mol��
��3����ʽ����������ˮ�������Fe��OH��2+�ɲ���ˮ�����ɾۺ�����Fe2��OH��42+����ˮ�ⷴӦ�����ӷ���ʽΪ2Fe��OH��2++2H2O?Fe2��OH��42++2H+��
��4����ҽҩ�ϳ����������������ᡢ����Ļ��Һ��Ӧ�Ʊ���ʽ�������������ҹ�����������Ʒ�в��ú���Fe2+��NO3-��Ϊ�������ò�Ʒ���Ƿ���Fe2+��Ӧʹ�õ��Լ�ΪD������ĸ����
A����ˮ B��KSCN��Һ C��NaOH��Һ D������KMnO4��Һ��
 ��
��