��Ŀ����
��֪Mg(OH)2��Al(OH)3�ǹ�ҵ�ϳ��õ���ȼ����Mg(OH)2�ķֽ��¶ȷ�ΧΪ340��490 �棬���������ķֽ��¶ȷ�ΧΪ190��230 �棬���ǵ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
Mg(OH)2(s)��MgO(s)��H2O(g)����H1����81.5 kJ��mol-1
Al(OH)3(s)��1/2Al2O3(s)��3/2H2O(g)����H2����87.7 kJ��mol-1
����˵������ȷ����
Mg(OH)2(s)��MgO(s)��H2O(g)����H1����81.5 kJ��mol-1
Al(OH)3(s)��1/2Al2O3(s)��3/2H2O(g)����H2����87.7 kJ��mol-1
����˵������ȷ����
[ ]
A��Mg(OH)2��Al(OH)3�����²��ֽ⣬���Կ�����ҵ��ȼ��
B��������Mg(OH)2��Al(OH)3��ȣ�Mg(OH)2��ȼЧ���Ϻ�
C��Mg(OH)2��Al(OH)3���ȶ��Ը�
D��Mg(OH)2��Al(OH)3��Ϊ��ҵ��ȼ�������Ƿֽ����ȼ�����������������й�
B��������Mg(OH)2��Al(OH)3��ȣ�Mg(OH)2��ȼЧ���Ϻ�
C��Mg(OH)2��Al(OH)3���ȶ��Ը�
D��Mg(OH)2��Al(OH)3��Ϊ��ҵ��ȼ�������Ƿֽ����ȼ�����������������й�
A

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ
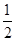 Al2O3(s)��
Al2O3(s)��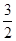 H2O(g)�� ��H2=��87.7kJ��mol��1
H2O(g)�� ��H2=��87.7kJ��mol��1