��Ŀ����
Ϊ��Ԥ����ȱ�������йز��Ź涨ʳ���еĵ⺬������I�ƣ�Ӧ��20~50 mg/Kg���Ʊ�KIO3�ķ������£�
����1��6I2��11KClO3��3H2O��6KH(IO3)2��5KCl��3Cl2����KH(IO3)2��KOH��2KIO3��H2O
����2�����������£�
����3��
���뷽��3��ȷ���1�IJ����� ������2�IJ��������� ��
�Ʒ���2ѡ�õĵ缫�Ƕ��Ե缫������������Ӧʽ�� ��
�Ƿ���3��Ӧ�¶ȿ�����70�����ң������ø����¶ȵ���Ҫԭ���� ��
���Ʊ�����KIO3��ʵ�鲽���У�����轫���þ������ʹ�� ϴ��2~3�Σ������ò�Ʒ��
����֪��KIO3��5KI��3H2SO4=3K2SO4��3I2��3H2O�� I2��2S2O32��=2I����S4O62����Ϊ�ⶨ�ӵ�ʳ���е�ĺ�������Ƶķ������¡�������ʵ�鲽�貢����ⶨ�����
I2��2S2O32��=2I����S4O62����Ϊ�ⶨ�ӵ�ʳ���е�ĺ�������Ƶķ������¡�������ʵ�鲽�貢����ⶨ�����
a��ȷ��ȡw gʳ������ƿ�У��ټ���������ˮʹ����ȫ�ܽ⡣
b�� ��
c������ƿ�еμ�2.0��10��3 mol/L Na2S2O3����Һ���յ㡣
d���ظ�����ʵ�����Ρ�
����ʵ������ݼ�¼���±���������ӵ�ʳ����Ʒ�еĵ�Ԫ�غ����� mg/kg���Ժ�w�Ĵ���ʽ��ʾ����
| �ζ����� | ʢ��Na2S2O3��Һ�Ķ��� | |
| �ζ�ǰ�̶ȣ�/mL�� | �ζ���̶ȣ�/mL�� | |
| 1 | 1.02 | 11.03 |
| 2 | 2.00 | 11.99 |
| 3 | 0.20 | 10.20 |
�ŷ���һ������������Ⱦ�����������Ĵ������ܣ�����1�֣�
��I��+ 6OH����6e��= IO3����3H2O ��2�֣�
��H2O2�ֽ⣬I2������������Ҫ������֣� ��2�֣�
�ȱ�ˮ����ˮ�Ҵ������������Ҵ����Һ�ȣ� ��2�֣�
����ϡ�����ữ������Һ���������KI��Һ���ٵμ�2-3�ε�����Һ�� ��2�֣�
1 270/3w
����

 KIO3��3H2�����������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
KIO3��3H2�����������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��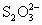 ��2I����
��2I���� )���ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________(��ϸ��ϸ�)��Ʒ��
)���ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________(��ϸ��ϸ�)��Ʒ��