��Ŀ����
��֪���Ը��������Һ���ԺͲ����ơ�������������ʷ���������ԭ��Ӧ��
(1)���Ը��������Һ�Ͳ����Ʒ�Ӧ�����ӷ���ʽΪ��MnO4-+C2O42-+H+��CO2��+Mn2++ H2O ��δ��ƽ�� �ֳ�ȡ������(Na2C2O4)��Ʒ1.34 g����ϡ�����У�Ȼ����0.20 mol/L�ĸ��������Һ�ζ������е����ʲ���������غ�ϡ���ᷴӦ�����ﵽ�յ�ʱ������15. 00 mL�ĸ��������Һ��
���жϵζ��ﵽ�յ�ʱ��������______________��
����Ʒ�в����Ƶ���������Ϊ______________��
(2)��д�����Ը��������Һ�Ͷ�������Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ___________
(1)���Ը��������Һ�Ͳ����Ʒ�Ӧ�����ӷ���ʽΪ��MnO4-+C2O42-+H+��CO2��+Mn2++ H2O ��δ��ƽ�� �ֳ�ȡ������(Na2C2O4)��Ʒ1.34 g����ϡ�����У�Ȼ����0.20 mol/L�ĸ��������Һ�ζ������е����ʲ���������غ�ϡ���ᷴӦ�����ﵽ�յ�ʱ������15. 00 mL�ĸ��������Һ��
���жϵζ��ﵽ�յ�ʱ��������______________��
����Ʒ�в����Ƶ���������Ϊ______________��
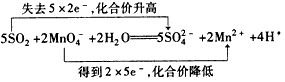
(2)��д�����Ը��������Һ�Ͷ�������Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ___________
(1)����Һ����ɫ��Ϊ�Ϻ�ɫ�����ڰ�����ڲ���ɫ����75%
(2)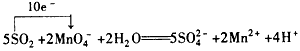 ��
��
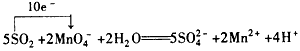 ��
��

��ϰ��ϵ�д�
�����Ŀ
 ������ͼ�ش����⣺
������ͼ�ش����⣺
 SO2��NOx����Ҫ�Ŀ�����ȾԴ�����뾭������������ŷţ�һ�ֹ�ҵβ����SO2��O2��N2��CO2��ijͬѧ����ͼʾװ�����ⶨ��β����SO2�ĺ�����
SO2��NOx����Ҫ�Ŀ�����ȾԴ�����뾭������������ŷţ�һ�ֹ�ҵβ����SO2��O2��N2��CO2��ijͬѧ����ͼʾװ�����ⶨ��β����SO2�ĺ�����