��Ŀ����
�����µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⡣
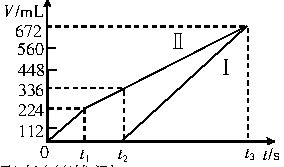
��1��ͨ�������Ʋ⣺
��ԭ�����Һ��NaCl��CuSO4�����ʵ���Ũ�ȡ�
��t2ʱ������Һ��pH��
��2��ʵ���з��֣���������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ���Լ�Ҫ��������ܵ�ԭ��
�� ����
�����������
����������Ĺؼ��Ƕ�ͼ��Ľ�����տ�ʼʱ����Cu2+�õ��ӣ�������ų���Cu2+��Ӧ�����Һ�е�H+��ʼ�ŵ�����H2����ˢ���672mLH2������������Һ�е�Cl-�ŵ�����Cl2����Ӧ�����Һ�е�OH-��ʼ�ŵ�����O2��������224mLCl2��448mLO2�����ݵ����غ�ã�2n��Cu2+��+2n��H2��=2n��Cl2��+4n��O2����
��1��������n��Cl-��=2��0.224L/22.4L?mol-1=0.0200mol������c��NaCl����0.0200mol/0.200L=0.100mol?L-1��
������2n��Cu2+��+2��0.672L/22.4L?mol-1��2��0.224L/22.4L?mol-1+4��0.448L/22.4L?mol-1��n��Cu2+����0.0200mol������c��CuSO4����0.0200mol/0.200L=0.100mol?L-1��
�ڸ�����Һ��H+��OH�������˳�ԭ��t2ʱ��Һ�е�n��H+��=4n�䣨O2��=4��0.00500mol=0.0200mol���������pH��1��
��2������������Cl2��O2��ˮ�е��ܽ�����Դ�������������H2��

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
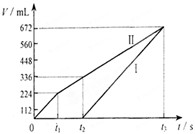 �����µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ��ͼ����ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�������м��㣺
�����µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ��ͼ����ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�������м��㣺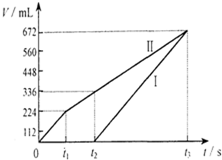 �����µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮
�����µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ����������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⣮

