��Ŀ����
(10��)ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�����ͼ��ʾ�ı仯��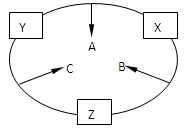
��֪B���������Zԭ�Ӹ�����C��������һ����
��ش��������⣺
(1) Ԫ��Xλ�� ���� ��
(2) Ԫ��Y��ԭ�ӽṹʾ��ͼ
(3) �õ���ʽ��ʾB���γɹ��̣�
(4) B��C���ȶ��Դ�С˳��Ϊ ���û�ѧʽ��ʾ��
(5) C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ
(1) �ڶ����ڢ�A�� ��2) 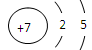
(3) 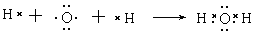
(4) H2O�� NH3 (5) 4NH3+5O2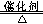 4NO+6H2O
4NO+6H2O
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ