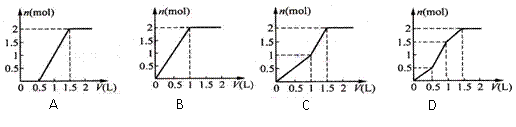
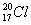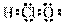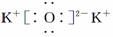��Ŀ����
(1) ��ͬ���ʵ�����O2��O3������������Ŀ֮��Ϊ_________,����O�����ʵ���֮��Ϊ_________��
(2) �ڱ�״���£�4 g H2��11.2 L O2��1 mol H2O�У���ԭ����������______�������С����_______��
(3) ����£���224L��HCl��������835ml����=1g/cm3����ˮ�У�����������ܶ�Ϊ1.2g/cm3��
����������ʵ���Ũ��___________
(4) ij������������Ϊ6.4 g������6.02��1022�����ӣ�����������Է�������Ϊ__________
(5)Na2CO3��Ħ��������____________,0.5mol Na2CO3��������____________,����________mol Na+, ______mol CO3 2-,Na+�ĸ���ԼΪ___________;����������ˮ,���250mL����Һ,�����Һ���ʵ����ʵ���Ũ��Ϊ_____________;������Һ�м��������ϡ����,�������������Ϊ(�����)__________.
��1��3:2 1:1
H2 H2O
��3��10 mol��L-1
��4��64
��5��106 g��mol-1 5.3 g 1 0.5 mol 6.02��1023 2 mol/L 1.12 L

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�