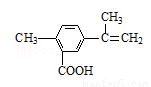
��Ŀ����
��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�������������Ӱ�ˮ���������ܽ�õ�����ɫ������Һ��������ɫ��Һ�м���������95%�Ҵ�������ɫ��Һ����ǣ����ú�������ɫ�������������жԴ�����˵����ȷ����
A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ��
B��[Cu(NH3)4]2+�Ŀռ乹��Ϊƽ�������Σ���������Cu2+����sp3�ӻ�
C��[Cu(NH3)4]SO4�����Ҵ�������Ӧ��������ɫ����
D��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3����
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ