��Ŀ����
20���������ڲ�ͬ�����¿���ת��Ϊ��ͬ���ʣ���ش��������⣺��֪��
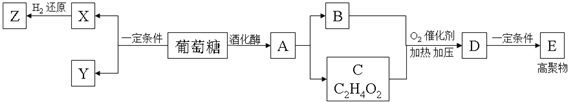
��1���������ھƻ�ø�������������л���A��B��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ�A��B��C��D��E���ת����ϵ����ͼ��
��д��D�й����ŵ�������������̼̼˫����
��д��A��B�Ļ�ѧ����ʽCH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
��E�Ľṹ��ʽCH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
��2����������һ�������¿�����ΪX��Y��X����Է�����������A��B֮�䣬Y����Է���������A��ͬ��X�ɴ�������Y��Ҳ����H2��Ӧ����Z��
������˵����ȷ����AD��
A�������£�������XΪ���� B�������������У����������Ǻͻ�����X���Է���������Ӧ
C��������A��Y��ͬ���칹�� D��������A��Z������ͬ�����ţ�����ͬϵ��
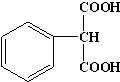
������������Է���������ͬ�ķ����廯����N�����ı�����ֻ��һ��ȡ������1mol N������NaHCO3��Ӧ������44.8L CO2����״��������N�Ľṹ��ʽΪ
 ��
��
���� �������ھƻ�ø�������������л���A��AΪCH3CH2OH��B��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ���BΪCH2=CH2��A������ȥ��Ӧ����B������C�ķ���ʽ��֪��A������CΪCH3COOH��B��C������Ϣ�еķ�Ӧ����DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ��EΪ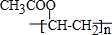 ����������һ�������¿�����ΪX��Y��X����Է�����������A��B֮�䣬Y����Է���������A��ͬ��X�ɴ�������Y����XΪHCHO��YΪHCOOH��X����H2��Ӧ����Z����ZΪCH3OH���ݴ˽��д��⣮
����������һ�������¿�����ΪX��Y��X����Է�����������A��B֮�䣬Y����Է���������A��ͬ��X�ɴ�������Y����XΪHCHO��YΪHCOOH��X����H2��Ӧ����Z����ZΪCH3OH���ݴ˽��д��⣮
��� �⣺�������ھƻ�ø�������������л���A��AΪCH3CH2OH��B��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ���BΪCH2=CH2��A������ȥ��Ӧ����B������C�ķ���ʽ��֪��A������CΪCH3COOH��B��C������Ϣ�еķ�Ӧ����DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ��EΪ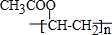 ����������һ�������¿�����ΪX��Y��X����Է�����������A��B֮�䣬Y����Է���������A��ͬ��X�ɴ�������Y����XΪHCHO��YΪHCOOH��X����H2��Ӧ����Z����ZΪCH3OH��
����������һ�������¿�����ΪX��Y��X����Է�����������A��B֮�䣬Y����Է���������A��ͬ��X�ɴ�������Y����XΪHCHO��YΪHCOOH��X����H2��Ӧ����Z����ZΪCH3OH��
��1����DΪCH3COOCH=CH2��D�й����ŵ�������������̼̼˫�����ʴ�Ϊ��������̼̼˫����
��A��B�Ļ�ѧ����ʽΪ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O���ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
�۸�������ķ�����֪��E�Ľṹ��ʽΪ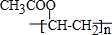 ���ʴ�Ϊ��
���ʴ�Ϊ��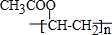 ��
��
��2����A��XΪHCHO�������£�HCHOΪ���壬��A��ȷ��
B�������������У��������Ǻ�HCHO��HCOOH�����Է���������Ӧ����B����
C��AΪCH3CH2OH��YΪHCOOH�����Dz���ͬ���칹�壬��C����
D��AΪCH3CH2OH��ZΪCH3OH����������ͬ�������ǻ�������ͬϵ���D��ȷ��
�ʴ�Ϊ��AD��
������������Է���������ͬ�ķ����廯����N������Է�������Ϊ180�����ı�����ֻ��һ��ȡ������1mol N������NaHCO3��Ӧ������44.8L CO2����״������2mol������ÿ��N���������Ȼ�����N�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϼ��л���ṹ�����ʣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ����ݷ�Ӧ���������ƶϣ���ȷ�ж����ʽṹ��ʽ�ǽⱾ��ؼ���ע��������Ϣ�����ã�����������ѧ���ķ���������������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ����Ӧ�ܵõ���ѧʽΪC7H5O3Na���ǣ�������
����Ӧ�ܵõ���ѧʽΪC7H5O3Na���ǣ�������| A�� | NaHCO3��Һ | B�� | Na2CO3��Һ | C�� | NaOH��Һ | D�� | NaCl��Һ |
| A�� | ���������ʱ�����ɵ��������������ӵ����� | |
| B�� | ���������ʱ�����ɵ������������������ӵ��Ǽ� | |
| C�� | ���������ʱ��ֻ���ɽ��������Ӻ���������ӵ����� | |
| D�� | NH4Cl�ĵ��뷽��ʽ��NH4Cl�TNH${\;}_{4}^{+}$+Cl-������NH4Cl���� |
| A�� | Na+��K+��SO42-��HCO3- | B�� | Cu2+��K+��SO42-��NO3- | ||
| C�� | Na+��K+��Cl-��SO42- | D�� | Na+��K+��NO3-��I- |
| A�� | x��ֵΪ4 | B�� | A��ת����Ϊ60% | ||
| C�� | B��ƽ��Ũ��Ϊ0.8 mol/L | D�� | D���������Ϊ20% |
| A�� | NA | B�� | 2NA | C�� | 1 | D�� | 2 |