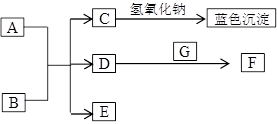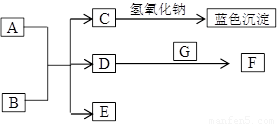��Ŀ����
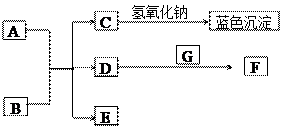
��8�֣���ͼ��A~G��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��GΪ�ǽ������ʣ�AΪ��ɫ���塢GΪ��̬��D��F���Ǵ�����Ⱦ���Ҫ��Դ������β��������DΪ����ɫ����ش��������⣺
��1��AԪ����Ԫ�����ڱ���λ�� ���� �� ��C�Ļ�ѧʽ��______________��
��2��д��D��E��Ӧת��ΪF��B�Ļ�ѧ����ʽ________________________________��
��3���ڳ����£�B��ϡ��ҺҲ����Cu��Ӧ����F���˷�Ӧ�����ӷ���ʽ��
��

���𰸡�
��8�֣��� �ڶ����2��1�֣� �ڢ�A��1�֣���CO2��2�֣�
�� 3NO2+H2O=2HNO3+NO��2�֣�û��ƽ��1�֣�
�� ���ӷ���ʽ 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����2�֣�д��ѧ����ʽ���÷֣�û��ƽ��1�֣�
��������

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ