��Ŀ����
�Ȼ���ͭ(CuCl)����Ҫ�Ļ���ԭ�ϡ����ұ��涨�ϸ�CuCl��Ʒ����Ҫ����ָ��ΪCuCl��������������96.50%����ҵ�ϳ�ͨ�����з�Ӧ�Ʊ�CuCl:2CuSO4+Na2SO3+2NaCl+Na2CO3====2CuCl��+3Na2SO4+CO2��(1)CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������Ƹ���Һ�����CuSO4��5H2O��H2O������֮�ȡ�
(2)ȷ��ȡ���Ʊ���0.250 0 g CuCl��Ʒ����һ������0.5 mol��L-1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20 mL����0.100 0 mol��L-1��Ce(SO4)2��Һ�ζ����յ㣬����24.60 mL Ce (SO4)2��Һ���йػ�ѧ��ӦΪ��
Fe3+CuCl====Fe2++Cu2++Cl-
Ce4++Fe2+====Fe3++Ce3+
ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
������(1) ����ҪCuSO4��5H2O������Ϊx��H2O������Ϊy
CuSO4��5H2O����Է�������Ϊ250��CuSO4����Է�������Ϊ160
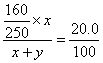
16x=5 (x+y) x:y=5:11
������CuSO4��5H2O��H2O������֮��Ϊ5��11��
(2) ����Ʒ��CuCl������Ϊx
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl��Fe2+��Ce4+
![]()
x=0.100 0 mol��L-1��24.60��10-3L��99.5 g��mol-1
x=0.244 8 g
![]() ��100%=97.92%
��100%=97.92%
97.92%��96.50%
����Ʒ��CuCl�������������ϱ���
�𰸣�(1) 5��11 (2) �ϸ�

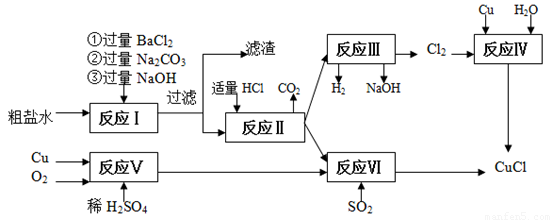
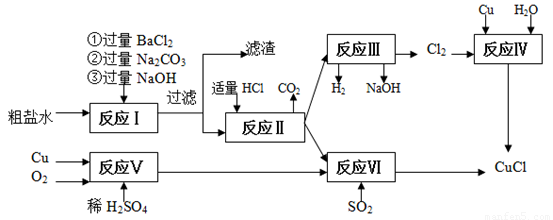
�Ȼ���ͭ(CuCl)�������л��ϳɹ�ҵ�еĴ�������һ�ְ�ɫ��ĩ������ˮ���������Ҵ���ϡ����ڿ�����Ѹ�ٱ���������ɫ��������ֽ⣬��ɺ�ɫ����ͼ�ǹ�ҵ��������ӡˢ��·�ķ�Һ����Fe3+��Cu2+��Fe2+��Cl-������CuCl���������£�


����������Ϣ�ش��������⣺
��1�����������̻��������ȼҵ�����Ṥҵ�������ϣ���ҵ��������ķ�����______________���ȼҵ��װ����_____________________��
��2��д������������X__________? Y___________ ���ѧʽ��
��3��д������CuCl�Ļ�ѧ����ʽ________________________________________________________��
��4��������Ϊ�����CuCl��Ʒ������������______________�����ٹ��ˣ�������CuCl���岻��ˮ������ˮ�Ҵ�ϴ�ӵ�Ŀ����______________________________�����������е�����Һ��pH���ܹ����ԭ����______________________________��
��5����CuCl�����ɹ����������ϲ���Ҫ����SO2���壬��������__________________________��
��6����CuCl�����ɹ����г��������⡢��ȫ�����⣬����Ϊ��Ӧ��ע��Ĺؼ�������:
_____________________________________��
��7���Ȼ���ͭ�Ķ���������
����ȡ��Ʒ0.25g(����0.0002g)����Ԥ�ȷ��벣����50����10ml������FeCl3��Һ250ml��ƿ�У�����ҡ�����������������____________________________��
������Ʒ�ܽ��ˮ50ml���ڷ�����ָʾ��2�Σ�
��������0.10 mol��L-1���������Һ������ɫ����Ϊ�յ㣻ͬʱ���հ�����һ�Ρ���֪��CuCl + FeCl3 =CuCl2 + FeCl2????? Fe2+ + Ce4+ = Fe3+ + Ce3+
������ظ����β�ã�
| 1 | 2 | 3 |
�հ�ʵ���������������Һ�����(ml) | 0.75 | 0.50 | 0.80 |
0.25����Ʒ�������������Һ�����(ml) | 24.65 | 24.75 | 24.70 |
�����ݴ����������CuCl�Ĵ���Ϊ____________����ƽ��ʵ�������ܳ���0.3%��