��Ŀ����
������KxFe��C2O4��y?3H2O��FeΪ+3�ۣ���һ�ֹ������ϣ�ʵ���ҿ��������µķ������Ʊ����ֲ��ϲ��ⶨ���ֲ��ϵ���ɣ�
���Ʊ�
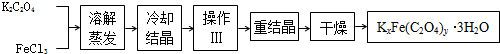
��1���ᾧʱӦ����Һ�ñ�ˮ��ȴ���ںڰ����ȴ����������������������ԭ���ǣ�
��2���������������
����ɲⶨ��ȡһ�������ľ���������ƿ�У���������������ˮ��ϡH2SO4����C2O42-ת��ΪH2C2O4����0.1000mol?L-1KMnO4��Һ�ζ���������KMnO4��Һ24.00mLʱǡ����ȫ��Ӧ��������Һ�м��������Ļ�ԭ����ǡ�ý�Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ4.00mL���ڶ��εζ������ӻ�ѧ����ʽΪ��
��3������100mL 0.1000mol?L-1KMnO4��Һ���ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ���
��4���û�����KxFe��C2O4��y?3H2O�У�x=
���Ʊ�

��1���ᾧʱӦ����Һ�ñ�ˮ��ȴ���ںڰ����ȴ����������������������ԭ���ǣ�
�ñ�ˮ��ȴ��������������ľ��壬�ڰ����Է�ֹ����ֽ�
�ñ�ˮ��ȴ��������������ľ��壬�ڰ����Է�ֹ����ֽ�
����2���������������
���ˡ�ϴ��
���ˡ�ϴ��
������ɲⶨ��ȡһ�������ľ���������ƿ�У���������������ˮ��ϡH2SO4����C2O42-ת��ΪH2C2O4����0.1000mol?L-1KMnO4��Һ�ζ���������KMnO4��Һ24.00mLʱǡ����ȫ��Ӧ��������Һ�м��������Ļ�ԭ����ǡ�ý�Fe3+��ȫת��ΪFe2+����KMnO4��Һ�����ζ�����Fe2+��ȫ����ʱ����ȥKMnO4��Һ4.00mL���ڶ��εζ������ӻ�ѧ����ʽΪ��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
��3������100mL 0.1000mol?L-1KMnO4��Һ���ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ���
100mL����ƿ����ʽ�ζ���
100mL����ƿ����ʽ�ζ���
�����������ƣ�����4���û�����KxFe��C2O4��y?3H2O�У�x=
3
3
��������I����1������Ŀ��Ϣ��֪��������KxFe��C2O4��y?zH2O��FeΪ+3�ۣ���һ�ֹ����в��ϣ���֪�ڰ���Ϊ�˷�ֹ�������ֽ⣮
��2��������ǰһ������ȴ�ᾧ���ᾧ����ȻҪ��������ĸҺ���룬������Ҫ���ˣ�������������������ʣ�ϴ�Ӻ��ٽ����ؽᾧ�ᴿ��
�ڶ��εζ������ӻ�ѧ����ʽΪ���������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ���ʽ��
��3����Ŀ����ȷ˵���������ʵ�����Һ���ƺ͵ζ�ʵ�飬������Ҫ����ƿ�͵ζ��ܣ��ؼ���Ҫȷд����100mL����ƿ������KMnO4��Һ��ǿ�����ԣ�������ʽ�ζ���ʢ�ţ�
��4����MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��֪��n��Fe3+��=5n��MnO4-������2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O��֪n��C2O42-��=
n��MnO4-�������ݻ�ѧʽ�������y��ֵ�����ݻ��ϼ۴�����Ϊ0����x��ֵ��
��2��������ǰһ������ȴ�ᾧ���ᾧ����ȻҪ��������ĸҺ���룬������Ҫ���ˣ�������������������ʣ�ϴ�Ӻ��ٽ����ؽᾧ�ᴿ��
�ڶ��εζ������ӻ�ѧ����ʽΪ���������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ���ʽ��
��3����Ŀ����ȷ˵���������ʵ�����Һ���ƺ͵ζ�ʵ�飬������Ҫ����ƿ�͵ζ��ܣ��ؼ���Ҫȷд����100mL����ƿ������KMnO4��Һ��ǿ�����ԣ�������ʽ�ζ���ʢ�ţ�
��4����MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��֪��n��Fe3+��=5n��MnO4-������2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O��֪n��C2O42-��=
| 5 |
| 2 |
����⣺I����1������Ŀ��Ϣ��֪��������KxFe��C2O4��y?zH2O��FeΪ+3�ۣ���һ�ֹ����в��ϣ���֪�ڰ���Ϊ�˷�ֹ�������ֽ⣬�ñ�ˮ��ȴ�����ܽ�ȣ���������������ľ��壻
�ʴ�Ϊ���ñ�ˮ��ȴ��������������ľ��壬�ڰ����Է�ֹ����ֽ⣮
��2��������ǰһ������ȴ�ᾧ���ᾧ����ȻҪ��������ĸҺ���룬������Ҫ���ˣ�������������������ʣ�ϴ�Ӻ��ٽ����ؽᾧ�ᴿ��
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
�ڶ��εζ������ӻ�ѧ����ʽΪ���������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ���ʽ����ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��3������100mL 0.1000mol?L-1KMnO4��Һ�������е�������֪������Ҫ100mL����ƿ�������е�������֪���ζ�ʵ�黹��Ҫ�ζ��ܣ�KMnO4��Һ��ǿ�����ԣ����Է�����Ƥ�ܣ�������ʽ�ζ���ʢ�ţ�
�ʴ�Ϊ��100mL����ƿ����ʽ�ζ��ܣ�
��4����MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��֪��n��Fe3+��=5n��MnO4-��=5��0.002L��0.10mol?L-1=0.001mol����2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O��֪��n��C2O42-��=
n��MnO4-��=
��0.012L��0.10mol?L-1=0.003mol������0.001mol��0.003mol=1��y�����y=3�����ݻ��ϼ۴�����Ϊ0��֪��x+3+3����-2��=0�����x=3��
�ʴ�Ϊ��3��
�ʴ�Ϊ���ñ�ˮ��ȴ��������������ľ��壬�ڰ����Է�ֹ����ֽ⣮
��2��������ǰһ������ȴ�ᾧ���ᾧ����ȻҪ��������ĸҺ���룬������Ҫ���ˣ�������������������ʣ�ϴ�Ӻ��ٽ����ؽᾧ�ᴿ��
�ʴ�Ϊ�����ˡ�ϴ�ӣ�
�ڶ��εζ������ӻ�ѧ����ʽΪ���������Һ�ζ��������ӷ���������ԭ��Ӧ�����ӷ���ʽ����ӦΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��3������100mL 0.1000mol?L-1KMnO4��Һ�������е�������֪������Ҫ100mL����ƿ�������е�������֪���ζ�ʵ�黹��Ҫ�ζ��ܣ�KMnO4��Һ��ǿ�����ԣ����Է�����Ƥ�ܣ�������ʽ�ζ���ʢ�ţ�
�ʴ�Ϊ��100mL����ƿ����ʽ�ζ��ܣ�
��4����MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��֪��n��Fe3+��=5n��MnO4-��=5��0.002L��0.10mol?L-1=0.001mol����2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O��֪��n��C2O42-��=
| 5 |
| 2 |
| 5 |
| 2 |
�ʴ�Ϊ��3��
���������⿼��ѧ���Թ����������⡢�Ķ���ȡ��Ϣ��������Һ���ơ�������ԭ��Ӧ�ζ������ȣ��Ѷ��еȣ���Ҫѧ���߱��Ķ������������������֪ʶ�Ľ������������

��ϰ��ϵ�д�
�����Ŀ



