��Ŀ����
ij��ʵ������480mL��0.5mol/L ��ϡH2SO4��Һ��ijͬѧ��98%��ŨH2SO4����=1.84g/cm3���������ƣ���ش��������⣺
��1��98%��ŨH2SO4����=1.84g/cm3�������ʵ���Ũ��Ϊ______
��2�������в����еĿո��ڽ���������������д����
����20mL��Ͳ��ȡ�����Ũ����
�ڽ��ձ��е���Һת�Ƶ�______mL������ƿ��
�۽�Ũ���Ỻ��ע��ʢ����������ˮ���ձ��У��ӱ߽���
�ܽ���Һ��ȴ���ָ�����
��������ƿ�м�������ˮ���ھ���̶�1��2cmʱ������______������ˮ���̶���
�Ǻ�ƿ�����������µߵ���ҡ��
��ϴ���ձ�2��3�Σ�ϴ��ҺҲע������ƿ�У�����ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�
��3��ʵ���������������ȷ˳��Ϊ______������ţ���
��4���ں�������д���и����������������ҺŨ�ȵ�Ӱ�죨ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�����õ�Ũ���᳤ʱ��������ܷⲻ�õ�������______
����ȡŨ����������Ͳ������ˮ______
�۶���ʱ������Һ��______��
�⣺��1��98%��ŨH2SO4����=1.84g/cm3�������ʵ���Ũ��Ϊ mol/L=18.4mol/L��
mol/L=18.4mol/L��
�ʴ�Ϊ��18.4mol/L��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ���Ũ�������ձ��ڱ�����ע��ʢ������ˮ���ձ��У��ӱ��ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У���������ƿ�м�����ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����ø��̶���ƽ���Ǻ�����ƿ���������ߵ�ҡ�ȣ�����õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ�����森
�ʢڽ��ձ��е���Һת�Ƶ� 500mL������ƿ�У���������ƿ�м�������ˮ���ھ���̶�1��2cmʱ������ ��ͷ�ιܼ�����ˮ���̶��ߣ�
�ʴ�Ϊ����500���ݽ�ͷ�ιܣ�
��3���ɣ�2���е�ʵ�鲽���֪��ʵ�������ȷ��˳��Ϊ���٢ۢܢڢߢݢޣ�
�ʴ�Ϊ���٢ۢܢڢߢݢޣ�
��4�������õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�Ũ������ˮ��Ũ�ȱ�ϡ��ʵ����ȡ��Ũ��������������Ķ����ʵ���ƫС��������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
����ȡŨ����������Ͳ������ˮ����ȡŨ�����ʵ�����ƫС�����䷴Ӧ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�۶���ʱ������Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��������1������c=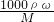 ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ�
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������û��480mL������ƿ��Ӧѡ�����480mL������������ƿ����ѡ��500mL������ƿ��
��3������ʵ������IJ������ʵ�鲽�������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=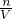 ����������������ҺŨ�ȵ�Ӱ�죮
����������������ҺŨ�ȵ�Ӱ�죮
���������⿼����һ�����ʵ���Ũ����Һ�����Ʋ��������Լ�����ѡ���������ȣ��ѶȲ�����c=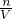 ������Һ����������������
������Һ����������������
 mol/L=18.4mol/L��
mol/L=18.4mol/L���ʴ�Ϊ��18.4mol/L��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ�Ũ���ᣬ���ձ���ϡ�ͣ���Ũ�������ձ��ڱ�����ע��ʢ������ˮ���ձ��У��ӱ��ò��������裬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У���������ƿ�м�����ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����ø��̶���ƽ���Ǻ�����ƿ���������ߵ�ҡ�ȣ�����õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ�����森
�ʢڽ��ձ��е���Һת�Ƶ� 500mL������ƿ�У���������ƿ�м�������ˮ���ھ���̶�1��2cmʱ������ ��ͷ�ιܼ�����ˮ���̶��ߣ�
�ʴ�Ϊ����500���ݽ�ͷ�ιܣ�
��3���ɣ�2���е�ʵ�鲽���֪��ʵ�������ȷ��˳��Ϊ���٢ۢܢڢߢݢޣ�
�ʴ�Ϊ���٢ۢܢڢߢݢޣ�
��4�������õ�Ũ���᳤ʱ��������ܷⲻ�õ������У�Ũ������ˮ��Ũ�ȱ�ϡ��ʵ����ȡ��Ũ��������������Ķ����ʵ���ƫС��������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
����ȡŨ����������Ͳ������ˮ����ȡŨ�����ʵ�����ƫС�����䷴Ӧ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�۶���ʱ������Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��������1������c=
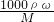 ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ���2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������û��480mL������ƿ��Ӧѡ�����480mL������������ƿ����ѡ��500mL������ƿ��
��3������ʵ������IJ������ʵ�鲽�������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=
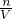 ����������������ҺŨ�ȵ�Ӱ�죮
����������������ҺŨ�ȵ�Ӱ�죮���������⿼����һ�����ʵ���Ũ����Һ�����Ʋ��������Լ�����ѡ���������ȣ��ѶȲ�����c=
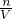 ������Һ����������������
������Һ���������������� 
��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ