��Ŀ����
����Ŀ��Ϊ��ʵ������ʵ��Ŀ�ģ������±��ṩ����Ҫ����,�����Լ��������ǣ� ��
ѡ�� | ʵ��Ŀ�� | ��Ҫ���� | �Լ� |
A | ����Br2��CCl4����� | ��Һ©�����ձ� | Br2��CCl4��������ˮ |
B | ̽�����۵�ˮ��̶� | �Թܡ��ձ����ƾ��� | ������Һ��������Һ |
C | ̽��ʯ���ͷֽ�����Ƿ��в������� | Ӳ���Թܡ��ƾ��ƣ�����̨������ | ʯ���͡����Ƭ�����Ը��������Һ |
D | �ⶨNaOH��ҺŨ�� | ��ʽ�ζ��ܡ����Եζ��ܡ���ƿ���ձ� | NaOH��Һ��0.1000mol/L���� |
A. A B. B C. C D. D
���𰸡�C
����������������CCl4����������ȡ����Һ������Br2��CCl4������A����̽�����۵�ˮ��̶ȵķ�����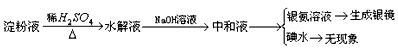 ��֤������ˮ����ȫ����B����
��֤������ˮ����ȫ����B����
ʯ���ͷֽ�����ϩ����������ϩ���к���̼̼˫������ʹ���Ը��������Һ��ɫ����C��ȷ��ȱָʾ���жϵζ��յ㣬��D����

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����������Ҫ���ܵ�ȼ�ϣ�ͨ������(N2H4)��Ϊȼ�ϣ�N2O4����������
��1����֪��N2(g)+2O2(g)=2NO2(g) ��H��+67.7kJ��mol-1
N2H4(g)+O2(g)=N2(g)+2H2O(g) ��H��-534.0kJ��mol-1
2NO2(g)![]() N2O4(g) ��H��-52.7kJ��mol-1
N2O4(g) ��H��-52.7kJ��mol-1
��д����̬��������������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��______________________��
��2����ҵ�Ͽ��ô�������������İ���Ӧ�Ʊ��£��÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ__________________��
��3����ҵ�ϳ��ð�ˮ������ҺpH��ȥ�������ӣ�������Cr(OH)3���ܶȻ�Ksp=10-32��ҪʹCr3+Ũ�Ƚ���10-5mol/L,��ҺpHӦ����____________________
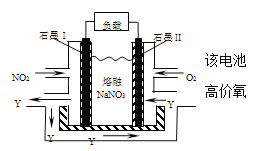
II����130���180��ʱ���ֱ�0.50molCH4��a mol NO2����1L���ܱ������з�����Ӧ��CH4(g)+2NO2(g) ![]() N2(g)+CO2(g)+2H2O(g) ��H��0������й��������±���
N2(g)+CO2(g)+2H2O(g) ��H��0������й��������±���
ʵ���� | �¶� | 0min | 10min | 20min | 40min | 50min | |
1 | 130�� | n(CH4)/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
2 | 180�� | n(CH4)/mol | 0.50 | 0.30 | 0.18 | 0.15 |
��4��180��ʱ�ﵽƽ��״̬ʱ��CH4��ƽ��ת����Ϊ___________________��
��5����֪130��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ6.4���Լ���a=__________��
��6��NO2��O2������NaNO3������ȼ�ϵ�أ���ԭ����ͼ����ʹ�ù�����ʯīI�缫������������Y (YΪ��Ԫ����ۻ�����)���õ缫��ӦΪ_________________��