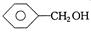
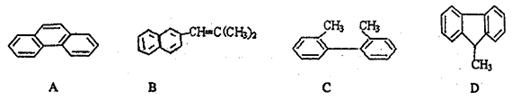
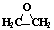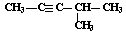��Ŀ����
���о����л���ټ��� �������� ����Ȳ �ܱ����� ��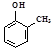 �ޱ� �ױ� ����ϩ ���Ҵ�
�ޱ� �ױ� ����ϩ ���Ҵ�
��1�����������������Ӧ�Ŀո��У�
�ճ���������ȼ�ϵ���___________������дһ�֣�����Ϊ��ȡըҩԭ�ϵ���________������дһ�֣���������������ˮ�����ӳɷ�Ӧ����_______________�������³�Һ̬��������ˮ����ȡ����Ҳ��ʹ���Ը��������Һ��ɫ����____________����Ϊͬϵ�����_______________��
��2���ݺ����ʵ�������___________________��
��3����������·���ʽ
�ڡ���__________________________________________________________________��
�����__________________________________________________________________��
ʵ������ȡ�۵ķ�Ӧ______________________________________________________��
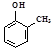 �ޱ� �ױ� ����ϩ ���Ҵ�
�ޱ� �ױ� ����ϩ ���Ҵ���1�����������������Ӧ�Ŀո��У�
�ճ���������ȼ�ϵ���___________������дһ�֣�����Ϊ��ȡըҩԭ�ϵ���________������дһ�֣���������������ˮ�����ӳɷ�Ӧ����_______________�������³�Һ̬��������ˮ����ȡ����Ҳ��ʹ���Ը��������Һ��ɫ����____________����Ϊͬϵ�����_______________��
��2���ݺ����ʵ�������___________________��
��3����������·���ʽ
�ڡ���__________________________________________________________________��
�����__________________________________________________________________��
ʵ������ȡ�۵ķ�Ӧ______________________________________________________��
(15��) (1) �ٻ�� �ܻ�� �ۢ� �� �ޢߣ���1�֣�
(2) �ڼ����ӻ�2-�����ӣ�1�֣�
(3) CH3CH2Br + NaOH CH2=CH2��+ NaBr + H2O��3�֣�
CH2=CH2��+ NaBr + H2O��3�֣�
CH3CH2OH + HBr CH3CH2Br + H2O��3�֣�
CH3CH2Br + H2O��3�֣�
CaC2 + H2O Ca(OH) 2 + CH��CH����3�֣�
Ca(OH) 2 + CH��CH����3�֣�
(2) �ڼ����ӻ�2-�����ӣ�1�֣�
(3) CH3CH2Br + NaOH
 CH2=CH2��+ NaBr + H2O��3�֣�
CH2=CH2��+ NaBr + H2O��3�֣�CH3CH2OH + HBr
 CH3CH2Br + H2O��3�֣�
CH3CH2Br + H2O��3�֣�CaC2 + H2O
 Ca(OH) 2 + CH��CH����3�֣�
Ca(OH) 2 + CH��CH����3�֣������������1���ճ���������ȼ�ϵ��Ǽ����״�����ѡ�ٻ���Ϊ��ȡըҩԭ�ϵ��Ǽױ����Ʊ�TNT������������Ʊ��������ͣ�����ѡ�ܻ�ߣ�������������ˮ�����ӳɷ�Ӧ������Ȳ����ϩ����ѡ�ۢࣻ�����³�Һ̬��������ˮ����ȡ����Ҳ��ʹ���Ը��������Һ��ɫ���Ǽױ�����ѡ�ߣ��ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ���л���ģ�����Ϊͬϵ����Ի�Ϊͬϵ����DZ��ͼױ�����ѡ�ޢߡ�
��2�����ݽṹ��ʽ��֪���ݺ����ʵ��������ڼ����ӻ�2-�����ӡ�
��3�������鷢����ȥ��Ӧ������ϩ���Ҵ����廯�ⷢ��ȡ����Ӧ�������������飻̼���ƺ�ˮ��Ӧ����������Ȳ����Ӧ�ķ���ʽ�ֱ���CH3CH2OH + HBr
 CH3CH2Br + H2O��CH3CH2Br + NaOH
CH3CH2Br + H2O��CH3CH2Br + NaOH CH2=CH2��+ NaBr + H2O ��CaC2 + H2O
CH2=CH2��+ NaBr + H2O ��CaC2 + H2O Ca(OH) 2 + CH��CH����
Ca(OH) 2 + CH��CH���������������ǻ���������Ŀ��飬���ض�ѧ������֪ʶ�Ĺ��̺�ѵ����ּ�ڹ���ѧ���Ļ��������ѧ��������û���֪ʶ���ʵ�����������������������ѧ���Ĺ淶�������������ѧ����Ӧ��������

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ


 ��
��