��Ŀ����
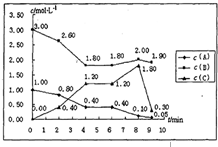
| ʱ��/���ʵ��� | n��A����mol�� | n��B����mol�� | n��C����mol�� | n��D����mol�� |
| ��ʼ | 0.9 | 1.4 | 0 | 0.2 |
| ��1min | 0.6 | 1.1 | 0.15 | |
| ��2min | 0.4 | 0.9 | 0.25 | |
| ��3min | 0.3 | 0.8 | 0.8 | |
| ��4min | 0.3 | 0.8 | 0.3 | 0.8 |
| ��5min | 0.35 | 0.85 | 0.275 | 0.75 |
��1���÷�Ӧ�Ļ�ѧ����ʽΪ��
��2����Ӧ����2minʱ����B��ʾ��ƽ����Ӧ����Ϊ
��3����Ӧ����3minʱ��A���ʵ�ת����Ϊ
��4��ʵ����������¶ȸ÷�Ӧ��ƽ�ⳣ��Kֵ���С����÷�ӦΪ
A������ B������B��Ũ�� C��ʹ�ô��� D��ͨ��ϡ������
��5��������˵���÷�Ӧ�ﵽƽ��״̬����
A����Ӧֹͣ�� B��2v��B������=v��C������
C����ϵ���ܶȱ��ֲ��� D����ϵ��ѹǿ���ֲ��䣮
��2������v=
| ��c |
| ��t |
��3�����ݷ�Ӧ����3minʱA�����ʵ�����������ĵ�A�����ʵ������ټ����ת���ʣ�
��4������ƽ�ⳣ������ʽ�������¶Ⱥ�ƽ�ⳣ���仯�ж����Ȼ��Ƿ��ȷ�Ӧ�����ݸ���ֵ����ʵ����仯�жϸı��������
��5�����ݴﵽƽ��״̬ʱ�����淴Ӧ������ȣ�����ֵ�Ũ�ȡ��ٷֺ�����������жϣ�
�ʴ�Ϊ��2A+2B?C+2D��
��2����Ӧ����2minʱ��B��Ũ�ȱ仯Ϊ��
| 1.4mol-0.9mol |
| 1L |
| 0.5mol/L |
| 2min |
�ʴ�Ϊ��0.25��
��3����Ӧ����3minʱ�����ĵ�A�����ʵ���Ϊ��0.9mol-0.3mol=0.6mol��A��ת����Ϊ��
| 0.6mol |
| 0.9mol |
�ʴ�Ϊ��66.7%��
��4�������¶�ƽ���������ȷ�Ӧ�����ƶ����¶����߸÷�Ӧ��ƽ�ⳣ��Kֵ���С��˵��ƽ�����������ƶ������Ը÷�ӦΪ���ȷ�Ӧ����4���ӵ���5���ӣ���Ӧ��Ũ������������Ũ�ȼ�С��˵��ƽ�����������ƶ���ʹ�ô�����ͨ��ϡ�����嶼��Ӱ��ƽ�⣬������B��Ũ�Ⱥ�A��Ũ�ȼ�С��C��D��Ũ��������Ҫ��ֻ�������¶ȣ�ƽ�����������ƶ�������Ҫ������A��ȷ��
�ʴ�Ϊ�����ȣ�A��
��5��A���ﵽƽ��״̬����Ӧû��ֹͣ��ֻ�����淴Ӧ������ȣ���A����
B��2v��B������=v��C����������ʾ�������淴Ӧ���ʣ����Dz����㻯ѧ��������ϵ�����淴Ӧ���ʲ���ȣ�û�дﵽƽ��״̬����B����
C����ϵ���ܶȱ��ֲ��䣬��Ӧ���嶼�����壬������������䣬�������ݻ����䣬���ݦ�=
| m |
| V |
D���÷�Ӧ�����������С�ķ�Ӧ����Ӧ��������������ʵ�����С����ϵ��ѹǿʼ�ձ仯������ϵ��ѹǿ���ֲ��䣬˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����D��ȷ��
��ѡ��D��

(13��)һ�������·�����Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��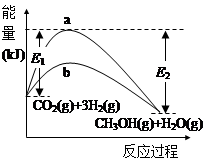
��1��ͼ������ (�a����b��)��ʾʹ�ô���ʱ�ķ�Ӧ���̡�ʹ�ô����Ը÷�Ӧ��Ӱ����______(��ѡ����ĸ)��
| A����߷�Ӧ���� | B�����CO2��ת���� |
| C�����ͷ�Ӧ��� | D���ı䷴Ӧ�Ħ�H |
 CH3OH(g)+H2O(g)�Ļ�ѧƽ�ⳣ���ı���ʽK=_________�������¶ȣ�Kֵ��_______(���������С�����䡱)��
CH3OH(g)+H2O(g)�Ļ�ѧƽ�ⳣ���ı���ʽK=_________�������¶ȣ�Kֵ��_______(���������С�����䡱)����3���ú�E1��E2�ı���ʽ��ʾCO2(g)+3H2(g)
 CH3OH(g)+H2O(g)�Ħ�H="_____" kJ��mol��1
CH3OH(g)+H2O(g)�Ħ�H="_____" kJ��mol��1��4��һ���¶��£������Ϊ2L���ݻ��̶����ܱ������У�����2molCO2��6molH2����10min��Ӧ�ﵽƽ��״̬W������1molCH3OH��CO2��ת����Ϊ________���ӷ�Ӧ��ʼ��ƽ�⣬��H2��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)=____________�����¶��£������Ϊ1L���ݻ��̶����ܱ������У����淴Ӧ��ʼ������ѧƽ�⣬�Ҹ���ֵ�ƽ��Ũ����ƽ��״̬W��ȫ��ͬ������ʼʱ����������n(CH3OH)=________��n(H2O)=________��
�ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ�������з�Ӧ��
2N2 (g)+6H2O (l)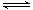 4NH3 (g)+3O2 (g)
��H�� a kJ��mol��1
4NH3 (g)+3O2 (g)
��H�� a kJ��mol��1
������ӦNH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
|
�¶� T/K |
303 |
313 |
323 |
|
NH3������/(10��6 mol) |
4.8 |
5.9 |
6.0 |
��1���˺ϳɷ�Ӧ��a 0 (�����������������)��
��2����ˮϡ��0.1 mol��L��1��ˮ����ϡ��ʱ��Һ�¶Ȳ��䣩������Һ������ˮ�������Ӷ���С�������е� ������ţ���
A��c(NH3��H2O)
B��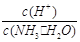 C��c(H+)��c(OH��)
D��
C��c(H+)��c(OH��)
D��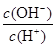
��3����ҵ�ð���ȡ�����������ӦΪ��4NH3(g)+5O2(g)
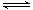 4NO(g)+6H2O(g)
��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
4NO(g)+6H2O(g)
��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
A B C D
��4����1L�ݻ��̶����ܱ������з���������Ӧ���������ʵ����ʵ���Ũ�����±���
|
ʱ��/Ũ�� |
c(NH3) (mol/L) |
c(O2 ) (mol/L) |
c(NO) (mol/L) |
|
��ʼ |
0.8000 |
1.600 |
0.000 |
|
��4 min |
0.3000 |
0.9750 |
0.5000 |
|
��6 min |
0.3000 |
0.9750 |
0.5000 |
|
��8 min |
0.7000 |
1.475 |
0.1000 |
��Ӧ�ڵ�6 min��8minʱ�ı����������ı������������___________________���ڸ������£�ƽ����_______�ƶ�(����ҡ�)��
 ��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ�� CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й����е������仯��