��Ŀ����
ijͬѧ��ȡ��4.0 gNaOH���壬������100 mL��Һ���ش��������⣺
(1)��ʵ���У��õ�����ȷ����������ֲ���������________��________��
(2)���������ж���õ�������������ʵ���е�������_____��
(3)���淶��ʵ������ᵼ��ʵ���������������в�����ʵ������Ӱ��(�ƫ����ƫС�����䡱)��
�����ܽ������������Һ�彦���ձ���________��
�ڶ���ʱ���������ϵĿ̶���________��
�۶��ݺ�����ƿ��ҡ�Ⱥ��÷���Һ����ڿ̶��ߣ������ּ�������ˮ���̶���________��
(4)������õ���ҺӦ������Լ�ƿ�У������ϱ�ǩ��������д�ñ�ǩ(��ͼ)��
(1)��ʵ���У��õ�����ȷ����������ֲ���������________��________��
(2)���������ж���õ�������������ʵ���е�������_____��
(3)���淶��ʵ������ᵼ��ʵ���������������в�����ʵ������Ӱ��(�ƫ����ƫС�����䡱)��
�����ܽ������������Һ�彦���ձ���________��
�ڶ���ʱ���������ϵĿ̶���________��
�۶��ݺ�����ƿ��ҡ�Ⱥ��÷���Һ����ڿ̶��ߣ������ּ�������ˮ���̶���________��
(4)������õ���ҺӦ������Լ�ƿ�У������ϱ�ǩ��������д�ñ�ǩ(��ͼ)��

��1����Ͳ ����ƿ ��2�����衢���� ��3��ƫС ƫС ƫС
��4��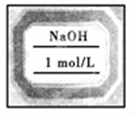
��4��

��1��ȷ����������ֲ�����������Ͳ������ƿ��
��2���ܽ�ʱ��Ҫ���裬ת��ʱ��Ҫ������
��3������c��n/V��֪�������ܽ������������Һ�彦���ձ��⣬�����ʼ��٣�Ũ��ƫС���ڶ���ʱ���������ϵĿ̶��ߣ�����Һ�����ƫ��Ũ��ƫС��ͬ��������Һ�����ƫ��Ũ��ƫС��
��4�����Լ��ı�ǩ��Ӧע���Լ��Ļ�ѧʽ�Լ����ʵ���Ũ�ȡ�
��2���ܽ�ʱ��Ҫ���裬ת��ʱ��Ҫ������
��3������c��n/V��֪�������ܽ������������Һ�彦���ձ��⣬�����ʼ��٣�Ũ��ƫС���ڶ���ʱ���������ϵĿ̶��ߣ�����Һ�����ƫ��Ũ��ƫС��ͬ��������Һ�����ƫ��Ũ��ƫС��
��4�����Լ��ı�ǩ��Ӧע���Լ��Ļ�ѧʽ�Լ����ʵ���Ũ�ȡ�

��ϰ��ϵ�д�
�����Ŀ
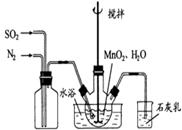
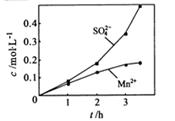
 ���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��
���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��