��Ŀ����
(8��)�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽ���£�

���ֲ��Ͽɲ�������ͼ��ʾ�ĺϳ�·��

(1)��
(2)RCOORl+R2OH��RCOOR2+R1OH(R��R1��R2��ʾ����)
�Իش��������⣺
(1)�ߵķ�Ӧ���������������� ����
(2)д��I�Ľṹ��ʽ�������������� ��
(3)�ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n (D)�� ����m��n��ʾ����
(4)д����Ӧ�ڵĻ�ѧ����ʽ����������������������������������������������
(5)д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ��
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��
���������������� ������������������ ������������������ ����

���ֲ��Ͽɲ�������ͼ��ʾ�ĺϳ�·��

(1)��

(2)RCOORl+R2OH��RCOOR2+R1OH(R��R1��R2��ʾ����)
�Իش��������⣺
(1)�ߵķ�Ӧ���������������� ����
(2)д��I�Ľṹ��ʽ�������������� ��
(3)�ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n (D)�� ����m��n��ʾ����
(4)д����Ӧ�ڵĻ�ѧ����ʽ����������������������������������������������
(5)д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ��
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��
���������������� ������������������ ������������������ ����
��1��ȡ����Ӧ
��2�� ��3��n�U(m+n)�Um
��3��n�U(m+n)�Um
��4��CH2Br��CH2Br��2NaOH 2NaBr��CH2OH��CH2OH
2NaBr��CH2OH��CH2OH
��5�� ��
�� ��
��
��2��
 ��3��n�U(m+n)�Um
��3��n�U(m+n)�Um��4��CH2Br��CH2Br��2NaOH
 2NaBr��CH2OH��CH2OH
2NaBr��CH2OH��CH2OH��5��
 ��
�� ��
��
�����л���ĺϳɡ�����PETG�Ľṹ��ʽ��֪�������������۲���䵥��ֱ����Ҷ������Զ�������� ����ΪE��B�������������E�ǶԶ������ᣬ��B�ǶԶ��ױ�����Ӧ����ȡ����Ӧ������F�Ľṹ��ʽΪ
����ΪE��B�������������E�ǶԶ������ᣬ��B�ǶԶ��ױ�����Ӧ����ȡ����Ӧ������F�Ľṹ��ʽΪ ��Fͨ��ˮ�ⷴӦ����G����G�ǶԶ����״���Gͨ���ӳɷ�Ӧ����H����H�Ľṹ��ʽΪ
��Fͨ��ˮ�ⷴӦ����G����G�ǶԶ����״���Gͨ���ӳɷ�Ӧ����H����H�Ľṹ��ʽΪ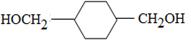 ������D���Ҷ��������A����ϩ���͵����巢���ӳɷ�Ӧ����C����C�Ľṹ��ʽΪCH2Br��CH2Br��C�پ���ˮ�ⷴӦ�������Ҷ���D��
������D���Ҷ��������A����ϩ���͵����巢���ӳɷ�Ӧ����C����C�Ľṹ��ʽΪCH2Br��CH2Br��C�پ���ˮ�ⷴӦ�������Ҷ���D��
��1������ȡ����Ӧ
��2������������Ϣ��2����֪I�Ľṹ��ʽΪ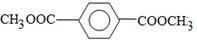 ��
��
��3������ԭ���غ��֪��n(H)��n(E)��n (D)��n�U(m+n)�Um��
��4����Ӧ����±������ˮ�ⷴӦ������ʽΪCH2Br��CH2Br��2NaOH 2NaBr��CH2OH��CH2OH��
2NaBr��CH2OH��CH2OH��
��5���ܷ���������Ӧ��˵������ȩ������ˮ�⣬˵���������������Ȼ���������ɫ��Ӧ��˵�����з��ǻ�������������ȡ����Ϊ��OH����CHO��HCOO������λ�ù���3�֣�������3��ͬ���칹�塣
 ����ΪE��B�������������E�ǶԶ������ᣬ��B�ǶԶ��ױ�����Ӧ����ȡ����Ӧ������F�Ľṹ��ʽΪ
����ΪE��B�������������E�ǶԶ������ᣬ��B�ǶԶ��ױ�����Ӧ����ȡ����Ӧ������F�Ľṹ��ʽΪ ��Fͨ��ˮ�ⷴӦ����G����G�ǶԶ����״���Gͨ���ӳɷ�Ӧ����H����H�Ľṹ��ʽΪ
��Fͨ��ˮ�ⷴӦ����G����G�ǶԶ����״���Gͨ���ӳɷ�Ӧ����H����H�Ľṹ��ʽΪ ������D���Ҷ��������A����ϩ���͵����巢���ӳɷ�Ӧ����C����C�Ľṹ��ʽΪCH2Br��CH2Br��C�پ���ˮ�ⷴӦ�������Ҷ���D��
������D���Ҷ��������A����ϩ���͵����巢���ӳɷ�Ӧ����C����C�Ľṹ��ʽΪCH2Br��CH2Br��C�پ���ˮ�ⷴӦ�������Ҷ���D����1������ȡ����Ӧ
��2������������Ϣ��2����֪I�Ľṹ��ʽΪ
 ��
����3������ԭ���غ��֪��n(H)��n(E)��n (D)��n�U(m+n)�Um��
��4����Ӧ����±������ˮ�ⷴӦ������ʽΪCH2Br��CH2Br��2NaOH
 2NaBr��CH2OH��CH2OH��
2NaBr��CH2OH��CH2OH����5���ܷ���������Ӧ��˵������ȩ������ˮ�⣬˵���������������Ȼ���������ɫ��Ӧ��˵�����з��ǻ�������������ȡ����Ϊ��OH����CHO��HCOO������λ�ù���3�֣�������3��ͬ���칹�塣

��ϰ��ϵ�д�
�����Ŀ
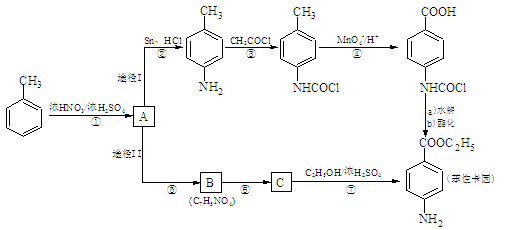
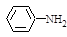 �������еİ����ױ�������
�������еİ����ױ�������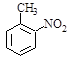 ��
��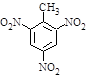 ��
��
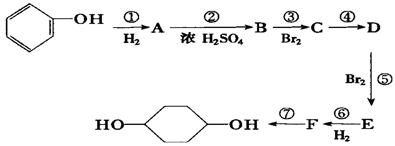
 �ϳ�
�ϳ�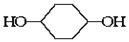 ��(�����Լ��ͷ�Ӧ��������ȥ)
��(�����Լ��ͷ�Ӧ��������ȥ) D��Ӧ�Ļ�ѧ����ʽ(�л���д�ṹ��ʽ����ע����Ӧ����)
D��Ӧ�Ļ�ѧ����ʽ(�л���д�ṹ��ʽ����ע����Ӧ����)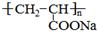 ����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ��������£�
����һ��ǿ��ˮ��֬����ij��A�ϳɾ۱�ϩ���Ƶ��������£�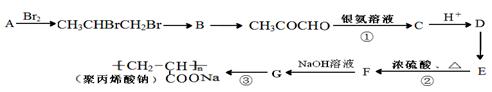
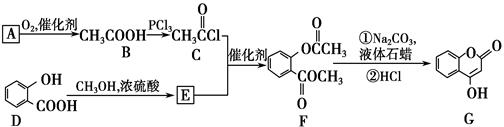
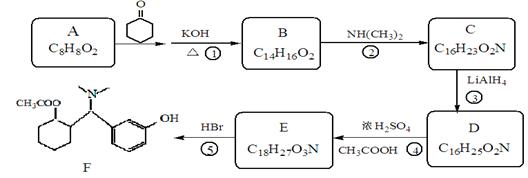
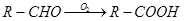
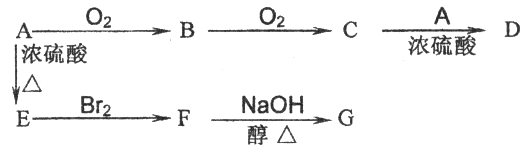
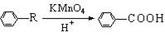 ��R��ʾ�뱽��ֱ��������̼ԭ��������ԭ�ӵ����������ִ�A������������ͼ��ʾ��һϵ�з�Ӧ������B��E�����ʵ���֮��1��2��Ӧ����W��
��R��ʾ�뱽��ֱ��������̼ԭ��������ԭ�ӵ����������ִ�A������������ͼ��ʾ��һϵ�з�Ӧ������B��E�����ʵ���֮��1��2��Ӧ����W��