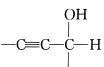
��Ŀ����
����Ŀ����Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣�����������γ����ꡢ�����Ȼ�����Ⱦ��������ף����ú��ʵĴ�ʩ��������Ⱦ�DZ�����������Ҫ��ʩ��
I���о���������NH3���������Ṥҵβ���е�NO��Ⱦ��NH3��NO�����ʵ���֮�ȷֱ�Ϊ1��3��3:1��4:1ʱ��NO�ѳ������¶ȱ仯��������ͼ��ʾ��

(1)������a�У�NO����ʼŨ��Ϊ6��10-4mg/m3����A �㵽B�㾭��0.8 s����ʱ�����NO���ѳ�����Ϊ____________mg��(m3��s)��
������b��Ӧ��NH3��NO�����ʵ���֮����____����������___________________
(2)��֪��25����101 kPaʱ��

��д����NH3�ѳ�NO���Ȼ�ѧ����ʽ��____________________________________
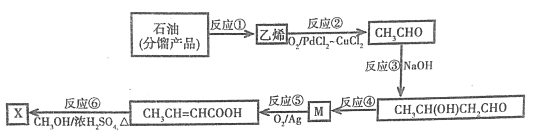
������ҵ�ϻ����Ա�������Ϊ���������������ﺬ�е�SO2��NO����Ⱦ��ת��ΪNa2S2O4(���շ�)��NH4NO3�Ȼ�����Ʒ����������������ͼ��

(3)װ������NOת��ΪNO3-�ķ�Ӧ�����ӷ���ʽΪ__________________________________
(4)װ����������ʹCe4+���������ü���ȼ�ϵ�ص���װ���е���Һ��������1 mol CH4 ʱ�������Ͽ�����____mol Ce4+��
���𰸡� 1.5��10-4 3:1 NH3��NO�����ʵ�����ֵԽ��NO���ѳ���Խ�� 4NH3(g)+6NO(g)�T5N2(g)+6H2O(1)��H=(3Q2-2Q1-3Q3)KJ/mol 3Ce4++NO+2H2O�T3Ce3++NO3-+4H+ 8
������������(1)������a�У�NO����ʼŨ��Ϊ6��10-4mg/m3��A����ѳ���Ϊ55%��B����ѳ���Ϊ75%����A�㵽B�㾭��0.8s����ʱ�����NO���ѳ�����=![]() =1.5��10-4mg/(m3s)���ʴ�Ϊ��1.5��10-4��
=1.5��10-4mg/(m3s)���ʴ�Ϊ��1.5��10-4��
��NH3��NO�����ʵ����ı�ֵԽ��NO�ѳ���Խ�������ʵ���֮�ȷֱ�Ϊ1��3��3��1��4��1ʱ����Ӧ������Ϊc��b��a��������b��ӦNH3��NO�����ʵ���֮����3��1���ʴ�Ϊ��3��1��NH3��NO�����ʵ�����ֵԽ��NO���ѳ���Խ��
(2)��N2(g)+3H2(g)�T2NH3(g)��H=-Q1/mol����2H2(g)+O2(g)�T2H2O(1)��H=-Q2kJ/mo1����N2(g)+O2(g)�T2NO(g)��H=+Q3kJ/mo1����NH3�ѳ�NO�Ļ�ѧ����ʽΪ4NH3(g)+6NO(g)�T5N2(g)+6H2O(1)���ɸ��ݸ�˹���ɣ���3����-2����-3�����ɵ��Ȼ�ѧ����ʽ4NH3(g)+6NO(g)�T5N2(g)+6H2O(1)��H=(3Q2-2Q1-3Q3)KJ/mol���ʴ�Ϊ��4NH3(g)+6NO(g)�T5N2(g)+6H2O(1)��H=(3Q2-2Q1-3Q3)KJ/mol��
����SO2��NO�Ǵ�����Ⱦ�ͨ������������Һ���յõ����������ƣ�һ����������Ӧ��ͨ��װ��������Ce4+������ԭ��Ӧ�õ�Ce3+��NO2-��NO3-�ȣ������������ƻ�ϣ��ڵ�����ͨ����õ�Ce4+ѭ��ʹ�ã����Na2S2O4��NO2-��NO3-�ȼ��백����������װ�����з�����Ӧ�õ�NH4NO3��Ʒ��
(3)װ������NOת��ΪNO3-�ķ�Ӧ�����ӷ���ʽΪ3Ce4++NO+2H2O�T3Ce3++NO3-+4H+���ʴ�Ϊ��3Ce4++NO+2H2O�T3Ce3++NO3-+4H+��
(4)����1 mol CH4 ת�Ƶ���8mol����������ͼ�����Խ�8mol Ce3+ת��ΪCe4+���ʴ�Ϊ��8��