��Ŀ����
��ͼ�Ǽء�����Ԫ���γɵ�һ�־����һ����������������С���ظ���Ԫ�������������Ļ��ϼۿɿ����Dz���Ϊ0�ۣ�����Ϊ��2�ۡ��ݴ˻ش�����С�⣺

1.�ýṹ�� �Ľṹ���ơ�
A��NaCl B��CsCl C���ɱ� D��SiO2
2.�ء�����Ԫ�����γɻ�����Ļ�ѧʽ��
A��K2O B��K2O2 C��K2O3 D��KO2
3.���ж�KO2����ṹ��������ȷ����
A����������ÿ��K�����������K����8��
B��������ÿ��K����Χ��8��O2����ÿ��O2����Χ��8��K��
C��ÿ�� O2����Χ����ҵȾ����K����Χ�ɵĿռ乹��Ϊ��������
D�������У�0����ԭ���룭2����ԭ�ӵ���Ŀ��Ϊ3:1
���𰸡�
1.A
2.D
3.C
��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ͼ�Ǽء�����Ԫ���γɵ�һ�־����һ����������������С���ظ���Ԫ�������������Ļ��ϼۿɿ����Dz���Ϊ0�ۣ�����Ϊ��2�ۡ��ݴ˻ش�����С�⣺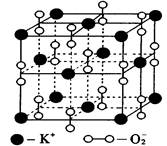
��С��1���ýṹ�� �Ľṹ���ơ�
| A��NaCl | B��CsCl | C���ɱ� | D��SiO2 |
| A��K2O | B��K2O2 | C��K2O3 | D��KO2 |
| A����������ÿ��K�����������K����8�� |
| B��������ÿ��K����Χ��8��O2����ÿ��O2����Χ��8��K�� |
| C��ÿ�� O2����Χ����ҵȾ����K����Χ�ɵĿռ乹��Ϊ�������� |
| D�������У�0����ԭ���룭2����ԭ�ӵ���Ŀ��Ϊ3:1 |






