��Ŀ����
��16�֣���֪��B������C��Hԭ�Ӹ�����Ϊ1�U2����Է�������Ϊ28�������µ�ת����ϵ��
 �ش�
�ش�
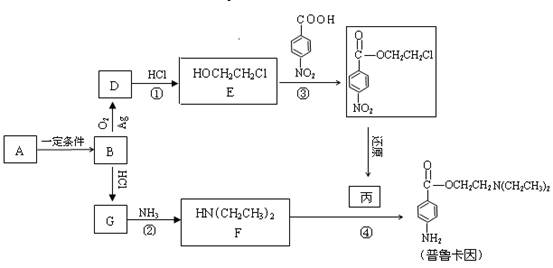
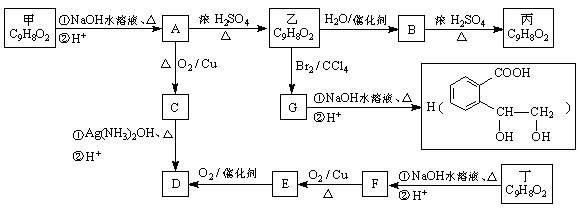
��1��A��������������������___________��
a���� b��±���� c���� d������
��2����Ӧ�ڿɱ�ʾΪ��G + NH3 �� F + HCl (δ��ƽ)���÷�Ӧ��ƽ��Ļ�ѧ����ʽ��
��
��3���۵ķ�Ӧ������_________________��
��4����³����������ˮ����ﶡ���졣
�����뻥Ϊͬ���칹��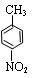 ����Ľṹ��ʽ��_____________________��
����Ľṹ��ʽ��_____________________��
��D�ĺ�����ױ��������ڳ�C��H����C��C�����������C��O���������ǻ�״�����д��D��F��һ�������·�Ӧ���ɶ��Ļ�ѧ����ʽ��
��
 �ش�
�ش���1��A��������������������___________��
a���� b��±���� c���� d������
��2����Ӧ�ڿɱ�ʾΪ��G + NH3 �� F + HCl (δ��ƽ)���÷�Ӧ��ƽ��Ļ�ѧ����ʽ��
��
��3���۵ķ�Ӧ������_________________��
��4����³����������ˮ����ﶡ���졣
�����뻥Ϊͬ���칹��
 ����Ľṹ��ʽ��_____________________��
����Ľṹ��ʽ��_____________________����D�ĺ�����ױ��������ڳ�C��H����C��C�����������C��O���������ǻ�״�����д��D��F��һ�������·�Ӧ���ɶ��Ļ�ѧ����ʽ��
��
��1��ab��4�֣���2��2CH3CH2Cl + NH3��NH(CH2CH3)2 +2HCl��3�֣�
��3��ȡ����Ӧ��������Ӧ��3�֣�
��4����
 ��3�֣�
��3�֣�
�� ��3�֣�
��3�֣�
��3��ȡ����Ӧ��������Ӧ��3�֣�
��4����
 ��3�֣�
��3�֣���
 ��3�֣�
��3�֣���

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д�
�����Ŀ
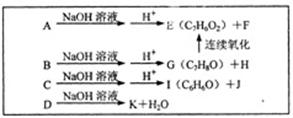
 ����Ӧ�IJ��ֲ�����ʡ�ԣ�����֪AΪ����Ԫ�أ�B�ڳ�����ΪҺ�壬E��GΪ��ȼ�����壬RΪ����ɫ
����Ӧ�IJ��ֲ�����ʡ�ԣ�����֪AΪ����Ԫ�أ�B�ڳ�����ΪҺ�壬E��GΪ��ȼ�����壬RΪ����ɫ ���壬K�����ʵ����ɫ��L��P����������Һ����������Ӧ������S�ķ���ʽΪC4H4O4�����������һ����Ԫ����TΪ�߷��ӻ����
���壬K�����ʵ����ɫ��L��P����������Һ����������Ӧ������S�ķ���ʽΪC4H4O4�����������һ����Ԫ����TΪ�߷��ӻ����
 ��Ư���� B�������� C����ԭ�� D������
��Ư���� B�������� C����ԭ�� D������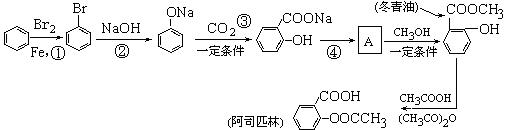
 ��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ��
��1��д������ת���ڵĻ�ѧ��Ӧ����ʽ�� ___________________________________________��
___________________________________________��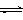 CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)��H2O(g) ����H����49.0kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��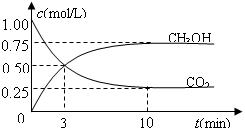
 ����CO����Ⱦ��������
����CO����Ⱦ�������� ���Ƿ���в�˵�����ɣ�����������������������������������������������������������������
���Ƿ���в�˵�����ɣ�����������������������������������������������������������������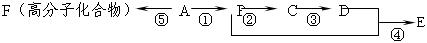


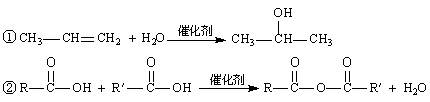

 �ṹ)����X�Ľṹ��ʽΪ (��дһ��)��
�ṹ)����X�Ľṹ��ʽΪ (��дһ��)��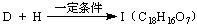 �������������I���� ��
�������������I���� ��