��Ŀ����
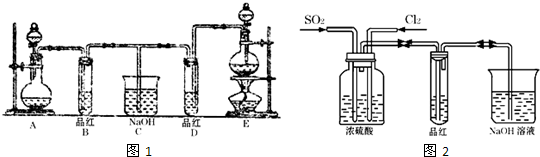
ʵ������Ũ���ᡢMnO2������ȡO2������CL2��Ca(OH)2��Ӧ������Ư�ۡ���֪��Ӧ2CL2+2Ca(OH)2=CaCL2+Ca(CLO)2+2H2O���¶��Ը�ʱ����������Ӧ6CL2+6Ca(OH)2=5CaCL2+6H2O����������ͬѧ�ֱ���Ƶ�����ʵ��װ������ͼ��ʾ��

(1) �����м�����ȱ�㣬���������װ�õ���ȱ������������ѡ�������ĿҪ���ѡ�������±��ո��ڡ�
a.�������Ʒ�Ӧ���ʣ�b�����Ʒ�Ӧ���ʣ�c�и���Ӧ������d�ɷ�ֹ����Ӧ������
e��Ⱦ������f�ɷ�ֹ��Ⱦ������
| �ŵ� | ȱ�� |
��װ�� |
|
|
��װ�� |
|
|
��װ�� |
|
|
(2) ͼʾװ���У�����A��B��������ɣ�����C��D��E��������ɣ�����F��G��������ɡ����ͼA�DG������װ����ѡȡ��������ɲ��֣���װ��һ��������ʵ��װ�ã���װ�ø����ֵ�����˳��(�����������ҵķ�����)___________________________��
(3) ʵ��������10ml��12mol?L-1��Ũ������������MnO2��Ӧ����������Ca(CLO)2�����ʵ�������С��0.015mol����ԭ����(�ٶ�������Ӧ����Ӧ�����ģ�������Ӧ����)_______________________________________��
(1) ��װ�ã�dװ�ã�d��ae����װ�ã�f��ac����װ�ã�b��ce��
(2) F ![]() B
B ![]() E
E
(3) Ũ������ϡ��������MnO2��Ӧ����������Ca(CLO)2������0.015mol.

������ͼ��ʾ���貢�ο��������ݣ��ش��������⣮
�����������������pH
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��2 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
��2���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ����������
��3�����ˢ�������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ����
��4�����ˢ�������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+�����õ�MnO2����Ӧ�����ӷ���ʽΪ
��5��ʵ���ҽ��й��˲���ʱ����Ҫ�õ��IJ���������
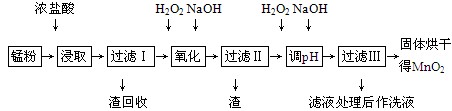
���յķϾ�п�̸ɵ�ؾ���������õ��̷�(��MnO2��Mn(OH)2��Fe����Ȳ�ͺ�̿��)�����̷���ȡMnO2�IJ�������ͼ��ʾ��
�����������������pH
|
���� |
��ʼ���� |
������ȫ |
|
Fe(OH)3 |
2.7 |
3.7 |
|
Fe(OH)2 |
7.6 |
9.6 |
|
Mn(OH)2 |
8.3 |
9.8 |
������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
(1)�ڼ�����������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2����Fe2���ȡ�MnO2��Ũ���ᷴӦ�����ӷ���ʽΪ___________________________________________
(2)�̷۾�Ũ�����ȡ�����ˢ��ȥ�������ʺ�����Һ�м�������H2O2��Һ����������____________________________________________________________��
(3)���ˢ�������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ����___________________________________________________________��
(4)���ˢ�������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2�������õ�MnO2����Ӧ�����ӷ���ʽΪ_____________________________________________��
(5)ʵ���ҽ��й��˲���ʱ����Ҫ�õ��IJ���������________________________