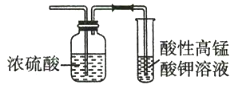
��Ŀ����
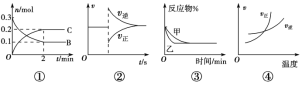
����Ŀ��(1)������0.1 mol��L��1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����________(����ĸ��ţ���ͬ)��
A��c(H��) B��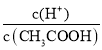 C��c(H��)��c(OH��) D��
C��c(H��)��c(OH��) D��![]()
������Һ�����¶ȣ�����4�ֱ���ʽ�������������________��
(2)ij�¶�ʱ��0.1 mol��L��1�Ĵ�����Һ�е�c(H��)��0.01 mol��L��1�Ĵ�����Һ�е�c(H��)�ı�ֵ______(����ڡ���С�ڡ����ڡ�)10��
(3)��֪��25 ��ʱ������ĵ���ƽ�ⳣ��Ϊ1.75��10��5��
������¶�ʱ��a mol��L��1�Ĵ�����Һ��c1(H��)��________mol��L��1(�ú�a�Ĵ���ʽ��ʾ)��[��ʾ����ʱa�Ƚ�С�����м��㣬ƽ��ʱc(CH3COOH)���ó�ʼŨ�ȴ��棬ˮ�������c(H��)��c(OH��)���Բ��ƣ���ͬ]
�������¶�ʱ�����Һ�м���һ������CH3COONH4(������Һ�������)��ʹ��Һ��c(CH3COO��)��Ϊb mol��L��1�����ʱc2(H��)��________(�ú�a��b�Ĵ���ʽ��ʾ)mol��L��1��
��c1(H��)________(����ڡ���С�ڡ����ڡ�)c2(H��)��
���𰸡�A ABC С�� ![]()
![]() ����
����
��������
(1)CH3COOH��Һ��ˮϡ�����У�������Һ�����������ĵ���̶Ƚ�С����ϡ�ͺ���Һ��c��H+����С����A��ȷ��B. ϡ�����������ӵ����ʵ�������������ʵ�����С��������ͬһ��Һ�У���Һ�����ͬ����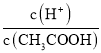 �ı�ֵ����B����C. ��Һ��c��H+��c��OH-��֮��Ϊˮ�����ӻ���ϡ�ͺ���Һ�¶Ȳ��䣬��ˮ�����ӻ����䣬��C����D. ϡ������������Ũ�ȼ�С������������Ũ��������
�ı�ֵ����B����C. ��Һ��c��H+��c��OH-��֮��Ϊˮ�����ӻ���ϡ�ͺ���Һ�¶Ȳ��䣬��ˮ�����ӻ����䣬��C����D. ϡ������������Ũ�ȼ�С������������Ũ��������![]() �ı�ֵ���D����ѡA��������Һ�����¶ȣ����ᡢˮ�ĵ���̶ȶ���������Һ�������ӡ�����������Ũ�ȶ�����A. �����¶Ⱥ���Һ��������Ũ��c��H+������A��ȷ��B.�����¶Ⱥ������ӡ�����������Ũ�ȶ�����
�ı�ֵ���D����ѡA��������Һ�����¶ȣ����ᡢˮ�ĵ���̶ȶ���������Һ�������ӡ�����������Ũ�ȶ�����A. �����¶Ⱥ���Һ��������Ũ��c��H+������A��ȷ��B.�����¶Ⱥ������ӡ�����������Ũ�ȶ�����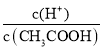 �������Ũ�ȼ�С����ñ�ֵ����B��ȷ��C.c��H+��c��OH-��Ϊˮ�����ӻ��������¶Ⱥ�ˮ�ĵ���̶�������ˮ�����ӻ�����C��ȷ��D.
�������Ũ�ȼ�С����ñ�ֵ����B��ȷ��C.c��H+��c��OH-��Ϊˮ�����ӻ��������¶Ⱥ�ˮ�ĵ���̶�������ˮ�����ӻ�����C��ȷ��D.![]() �������¶Ⱥ����������ӡ�������Ũ�ȶ���������������Ũ������ķ��ȴ������������ӣ����Ըñ�ֵ��С����D���ʴ�Ϊ��ABC��
�������¶Ⱥ����������ӡ�������Ũ�ȶ���������������Ũ������ķ��ȴ������������ӣ����Ըñ�ֵ��С����D���ʴ�Ϊ��ABC��
(2)����Һ��Ũ��ԽС����̶�Խ�����0.1mol/L��CH3COOH��Һ��0.01mol/L��CH3COOH��Һ�е�c��H+��֮��С��10���ʴ�Ϊ��С�ڣ�
(3)��c��H+��=![]() =
=![]() mol/L��
mol/L��
��c��H+��=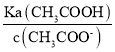 =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
�ۼ���һ������CH3COONH4��������Һ������䣩����������Ũ�����������ƽ�������ƶ���������Ũ�ȼ�С������c1��H+����c2��H+����

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�����Ŀ����һ���¶��£�������X������Y��0.16mol����10L�����ܱ������У�������Ӧ
X(g)��Y(g) ![]() 2Z(g) ��H < 0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���
2Z(g) ��H < 0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 7 | 9 |
n(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
����˵����ȷ����
A����Ӧǰ2min��ƽ�����ʦ�(Z)=2.0��10��3mol��L��1��min-1
B�������������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰ��(��)> ��(��)
C�����¶��´˷�Ӧ��ƽ�ⳣ��K=1.44
D�� �����������䣬�ٳ���0.2molZ��ƽ��ʱX�������������