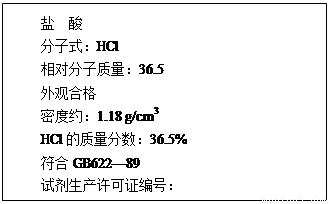��Ŀ����
��ͼΪij���������Լ�ƿ��ǩ�ϵIJ������ݡ��ʣ�
| �� �� ����ʽ��HCl ��Է���������36.5 ��ۺϸ� �ܶ�Լ��1.18 g/cm3 HCl������������36.5% ����GB622~89 �Լ���������֤��ţ� |
��1������������ʵ���Ũ��Ϊ���٣�����ʽ���㣩
��2��ȡ������25.5 mL��2.00 mol/L������������Һ100 mL��ϣ��ٽ���Ϻ���Һϡ����1.00 L����ʱ��Һ��pHԼΪ���٣�
��������1)c(HCl)=1000 mL��1.18 g/cm3��36.5%/36.5 g/mol��1 L=11.8 mol/L��
��2)n(HCl)=11.8 mol/L��0.0255 L��0.300 mol,n(NaOH)=2.00 mol/L��0.100 L=0.200 mol,
��ϲ�ϡ�ͺ���Һ��c(H+)=0.300 mol-0.200 mol/1.00 L=0.100 mol/L,pH=-lgc(H+)=1.
�𰸣���1)c(HCl)=1000 mL��1.18 g/cm3��36.5%/36.5 g/mol��1 L=11.8 mol/L
��2)1

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
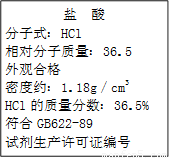
��ʦָ��һ��ͨϵ�д��� �� ����ʽ��HCl ��Է���������36.5 ��ۺϸ� �ܶ�Լ�� HCl������������36.5% ����GB622-89 �Լ���������֤��ţ� |
(1)����������ʵ���Ũ��Ϊ���٣�(��ʽ����)
(2)ȡ������25.4 mL��2.00 mol��L-1������������Һ100 mL��ϣ��ٽ���Ϻ���Һϡ����