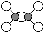
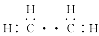ƒøƒ⁄»ð
”√ ؔա—ªØ∫Õ¡—Ω‚π˝≥õ√µΩµƒ““œ©°¢±˚œ©¿¥∫œ≥…±˚œ©À·““ı•µƒ¬∑œþ»Áœ¬:
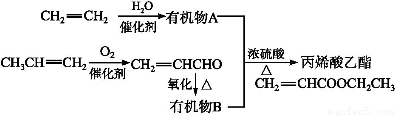
∏˘æð…œÕº∫Õƒ„À˘—ßµƒªØ—ß÷™ ∂ªÿ¥œ¬¡–Œ Â:
(1)”…CH2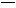 CH2÷∆µ√”–ª˙ŒÔAµƒªØ—ß∑Ω≥Ã ΩŒ™°°°°°°°°°°°°°°°°°°,∑¥”¶¿ý–Õ «°°°°°°°°°£
CH2÷∆µ√”–ª˙ŒÔAµƒªØ—ß∑Ω≥Ã ΩŒ™°°°°°°°°°°°°°°°°°°,∑¥”¶¿ý–Õ «°°°°°°°°°£
(2)±˚œ©(CH3CH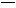 CH2)÷–∫¨”–µƒπŸƒÐÕ≈°°°°°°°°(ÃÓ√˚≥∆)°£
CH2)÷–∫¨”–µƒπŸƒÐÕ≈°°°°°°°°(ÃÓ√˚≥∆)°£
(3)A”ÎB∫œ≥…±˚œ©À·““ı•µƒªØ—ß∑¥”¶∑Ω≥Ã Ω «°°°°°°°°°°°°°°°°°°°°°°°°°£∏√∑¥”¶µƒ¿ý–Õ «°°°°°°°°°£
(4)”… ؔա—Ω‚≤˙ŒÔ““œ©∫œ≥…怓“œ©ÀСœµƒªØ—ß∑Ω≥Ã Ω «°°°°°°°°°°°°°°°°°°°°°°°°°£
(1)CH2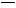 CH2+H2O
CH2+H2O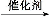 CH3CH2OH(1∑÷)°°º”≥…∑¥”¶(1∑÷)
CH3CH2OH(1∑÷)°°º”≥…∑¥”¶(1∑÷)
(2)úúÀ´º¸(1∑÷)
(3)CH2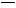 CHCOOH+CH3CH2OH
CHCOOH+CH3CH2OH CH2
CH2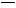 CHCOOCH2CH3+H2O(2∑÷)
CHCOOCH2CH3+H2O(2∑÷)
»°¥˙∑¥”¶(ªÚı•ªØ∑¥”¶)(1∑÷)
(4)nCH2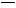 CH2
CH2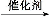

¡∑œ∞≤·œµ¡–¥∞∏
 ø⁄À„Âø®±±æ©∏æ≈Æ∂˘ÕØ≥ˆ∞Ê…Áœµ¡–¥∞∏
ø⁄À„Âø®±±æ©∏æ≈Æ∂˘ÕØ≥ˆ∞Ê…Áœµ¡–¥∞∏
œýπÿƒø