��Ŀ����
�� 4 mol A ����� 2 mol B ������ 2 L �Ķ��������л�ϲ���һ�������·������·�Ӧ �� ���� 2 s���� C �����ʵ���Ũ��Ϊ 0.6 mol��L-1���������м���˵����
���� 2 s���� C �����ʵ���Ũ��Ϊ 0.6 mol��L-1���������м���˵����
�� ������ A ��ʾ�����ʱ���ƽ������Ϊ 0.3 mol��L-1��s-1
�� ������ B ��ʾ�����ʱ���ƽ������Ϊ 0.6 mol��L-1��s-1
�� 2 s ʱ���� A ��ת����Ϊ30%
�� 2 s ʱ���� B �����ʵ���Ũ��Ϊ 0.3mol��L-1
������ȷ����
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
A
�������� 2 s���룩���� C ��Ũ��Ϊ 0.6mol��L��1 ����μӷ�Ӧ��AΪ1.2molBΪ0.6mol����C1.2mol VA="0.3" mol��L��1��s��1 VB=0.15mol��L��1��s��1 ����ȷ�ڴ���2 s ʱ���� A��ת����1.2/4=0.3������ȷ��2 s ʱ���� B��Ũ��Ϊ0.7mol��L��1���ܴ����ۺϺ�ɵ�ѡA��

��ϰ��ϵ�д�
�����Ŀ
�� 4 mol A�����2 mol B������2 L�������л�ϲ���һ�������·������·�Ӧ��2A��g����B��g��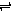 2C��g������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol?L��1��
2C��g������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol?L��1��
����������ȷ����
| A�������� B ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.6 mol?L��1?s��1 |
| B��2 s ʱ���� B ��Ũ��Ϊ 0.7 mol?L��1 |
| C��2 s ʱ���� A ��ת����Ϊ70�� |
| D�������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.3 mol?L��1?s��1 |
 2C(g)������2 s����C��Ũ��Ϊ0.6 mol��L��1����
2C(g)������2 s����C��Ũ��Ϊ0.6 mol��L��1����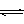 2C(g)������2 s����C��Ũ��Ϊ0.6 mol/L���������м���˵����
2C(g)������2 s����C��Ũ��Ϊ0.6 mol/L���������м���˵����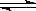 2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����
2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����������ȷ����