题目内容
【题目】(1)已知2 mol氢气燃烧生成液态水时放出572 kJ热量,反应方程式是
2H2(g)+O2(g)==2H2O(l)
(l)请回答下列问题:
①该反应的生成物能量总和________(填“大于”、“小于”或“等于”)反应物能量总和。
②若2 mol氢气完全燃烧生成水蒸气,则放出的热量________(填“>”、“<”或“=”)572 kJ。
③与化石燃料相比,利用氢能源有很多优点,请说出其中一点______________________。
(2)FeS2焙烧产生的SO2可用于制硫酸。已知25 ℃、101 kPa时:
2SO2(g)+O2(g)![]() 2SO3(g) ΔH1=-197 kJ·mol-1;
2SO3(g) ΔH1=-197 kJ·mol-1;
H2O(g)===H2O(l) ΔH2=-44 kJ·mol-1;
2SO2(g)+O2(g)+2H2O(g)===2H2SO4(l)ΔH3=-545 kJ·mol-1
则SO3(g)与H2O(l)反应生成H2SO4(l)的热化学方程式是_________________________________________。
【答案】(1)小于;<;热值高;清洁,无污染。(其它合理答案均可给分)
(2)SO3(g)+H2O(l)===H2SO4(l) ΔH=-152 kJ·mol-1;
【解析】
试题分析:(1)①因放热反应中生成物能量总<与反应物能量总和,而2H2(g)+O2(g)═2H2O(l)△H=-572kJ/mol是放热反应。
故答案为:小于;
②据热化学方程式2H2(g)+O2(g)═2H2O(l) △H=-572kJ/mol,2mol氢气完全燃烧生成液态水放出热量572kJ,因液态水变成水蒸气需要吸热,所以2mol氢气完全燃烧生成水蒸气放出热量小于572kJ。
故答案为:<;
③与化石燃料相比,利用氢能源有很多优点,燃烧产物为水无污染,燃烧热值高;
故答案为:热值高;清洁,无污染;
(2)2SO2(g)+O2(g)=2SO3(g) △H1=一197kJ/mol ①
2H2O(g)=2H2O(l) △H2=-44kJ/mol ②
2SO2(g)+O2(g)+2H2O(g)=2H2SO4(l) △H3=一545kJ/mol③
利用盖斯定律:(③-①-②)×1/2得SO3(g)+H2O(l)=H2SO4(l) △H=-152kJ/mol,
SO3(g)+H2O(l)===H2SO4(l) ΔH=-152 kJ·mol-1。
故答案为:SO3(g)+H2O(l)===H2SO4(l) ΔH=-152 kJ·mol-1;

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案【题目】某研究性学习小组为了探究醋酸的电离情况,进行了如下实验。
探究浓度对醋酸电离程度的影响
用pH计测定25 ℃时不同浓度的醋酸的pH,其结果如下:
醋酸浓度/(mol/L) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
回答下列问题:
(1)写出醋酸的电离方程式:___________________________________________。
(2)醋酸溶液中存在的微粒有________________________________________。
(3)根据表中数据,可以得出醋酸是弱电解质的结论,你认为得出此结论的依据是________________________________________________________________________。
(4)从表中的数据,还可以得出另一结论:随着醋酸浓度的减小,醋酸的电离程度将(填“增大”、“减小”或“不变”)__________。
【题目】乙醇是一种非常重要的烃的衍生物,是无色有特殊气味的液体。某校化学兴趣小组对乙醇的结构和性质进行了以下探究,请你参与并完成对有关问题的解答。
【观察与思考】
(1)乙醇分子的核磁共振氢谱有 个吸收峰。
【活动与探究】
(2)甲同学向小烧杯中加入无水乙醇,再放入一小块金属钠(约绿豆粒大),观察实验现象。请在下表中将观察到的实验现象及结论补充完全(有多少现象等就填多少,不必填满)。
实验现象 | 结 论 | |
① | 钠沉在乙醇下面 | 钠的密度大于乙醇 |
② |
(3)乙同学向试管中加入3~4 mL无水乙醇,浸入50℃左右的热水中,再将铜丝烧至红热,迅速插入乙醇中,反复多次。则此时乙醇发生反应的化学方程式为(生成乙醛)____________________________________。欲验证此实验的有机产物,可以将产物加入盛有 的试管中并在酒精灯火焰上直接加热,观察现象即可,此反应的化学方程式为__________________________________________。
【交流与讨论】
(4)丙同学向一支试管中加入3 mL 乙醇,然后边摇动试管边慢慢加入2 mL浓硫酸和2 mL冰醋酸,按右图所示连接好装置,请指出该装置的主要错误是 。假如乙醇分子中的氧原子为18O原子,则发生此反应后1 8O原子将出现在生成物 中(填字母)。
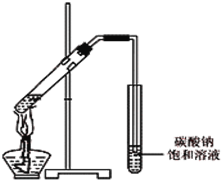
A.水 B.乙酸乙酯 C.水和乙酸乙酯
【题目】实验室中某些气体的制取、收集及尾气处理装置如图所示(省略夹持和净化装置)。仅用此装置和表中提供的物质完成相关实验,最合理的选项是( )

选项 | a中的物质 | b中的物质 | c中收集的气体 | d中的物质 |
A | 浓氨水 | CaO | NH3 | H2O |
B | 浓硫酸 | Na2SO3 | SO2 | NaOH溶液 |
C | 稀硝酸 | Cu | NO2 | H2O |
D | 稀硫酸 | CaCO3[ | CO2 | NaOH溶液 |

