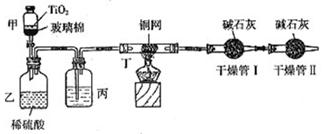
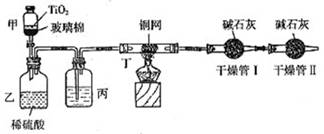
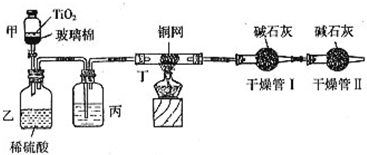
��Ŀ����
���ݻ���Ҫ���ڴ��������Ʒ�ˮʱ���綾��CN-�����ڴ���TiO2���������£�����NaClO��CN-����������CNO-��CN-��CNO-��NԪ�ؾ�Ϊ-3�ۣ����������������¼�����NaClO��Ӧ����N2��CO2��Cl2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬�ⶨCN-�������İٷ��ʣ��ֽ�Ũ����CN-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN-��Ũ��Ϊ0.2mol/L��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺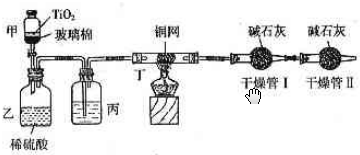
��1���������⣬д���й���Ҫ��Ӧ�����ӷ���ʽ��
���еķ�Ӧ��______��
���еķ�Ӧ��______��
��2������ʵ����ͨ���ⶨCO2������ȷ����CN-�Ĵ���Ч����
��װ���е��Լ���______����װ�õ�Ŀ����______������ܢ��������______������ܢ��������______��
��3���ٶ�����ÿһ��װ�ö�������գ������ø�װ�ò�õ�CN-�������İٷ�����ʵ��ֵ���______�����ƫ�ߡ���ƫ�͡������������ܵ�ԭ��______��
��4��������ܢ��м�ʯ������1.408g�����ʵ���в��CN-�������İٷ���Ϊ______��
�⣺��1�����������֪�����еķ�ӦΪNaClO��CN-����������CNO-��NaClO����ǿ�����ԣ�����ԭΪCl-����Ӧ���ӷ���ʽΪCN-+ClO-�TCNO-+Cl-�����еķ�ӦΪ����������CNO-��NaClO��Ӧ����N2��CO2��Cl2����Ӧ���ӷ���ʽΪ2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O��
�ʴ�Ϊ��CN-+ClO-�TCNO-+Cl-��2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O��
��2��ʵ��ԭ��Ϊ��������ܢ����յĶ�����̼������ȷ����CN-�Ĵ�����������װ���в���N2��CO2��Cl2��H2O��������ˮ���ܱ���ʯ�����գ�Ӱ�������̼�����IJⶨ�����Խ������ܢ������Ӧ��ȥ������ˮ����Ũ������ˮ����ͭ����ȥ������ͬʱӦ��ֹ�����е�CO2��ˮ�����������ܢ����ʵ�飮�ʱ�װ���е��Լ���Ũ�����װ�õ�Ŀ���dz�ȥCl2������ܢ������������CO2������ܢ�������Ƿ�ֹ�����е�CO2��ˮ�����������ʵ�飮
�ʴ�Ϊ��Ũ���ᣬ��ȥCl2������CO2����ֹ�����е�CO2��ˮ�����������ʵ�飮
��3��װ���в�����CO2δ����ȫ���գ����²ⶨ�Ķ�����̼����ƫ�ͣ���õ�CN-�������İٷ�����ʵ��ֵ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�װ���в�����CO2δ����ȫ���գ�
��4������ܢ��м�ʯ������1.408gΪ������̼�����������ʵ���Ϊ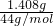 =0.032mol������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2��=0.032mol��ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol�����Ը�ʵ���в��CN-�������İٷ���Ϊ
=0.032mol������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2��=0.032mol��ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol�����Ը�ʵ���в��CN-�������İٷ���Ϊ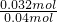 ��100%=80%��
��100%=80%��
�ʴ�Ϊ��80%
��������1�����������֪�����еķ�ӦΪNaClO��CN-����������CNO-�����еķ�ӦΪ����������CNO-��NaClO��Ӧ����N2��CO2��Cl2������������ԭ��Ӧ��ƽ��
��2��ʵ��ԭ��Ϊ��������ܢ����յĶ�����̼������ȷ����CN-�Ĵ�����������װ���в���N2��CO2��Cl2��H2O��������ˮ���ܱ���ʯ�����գ�Ӱ�������̼�����IJⶨ�����Խ������ܢ������Ӧ��ȥ������ˮ����Ũ������ˮ����ͭ����ȥ������ͬʱӦ��ֹ�����е�CO2��ˮ�����������ܢ����ʵ�飮
��3��װ���в�����CO2δ����ȫ���գ����²ⶨ�Ķ�����̼����ƫ�ͣ�
��4������ܢ��м�ʯ������1.408gΪ������̼������������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2����ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol���ݴ˼��㣮
����������ʵ��ԭ����ʵ��װ�����������ۡ����û�ѧ�����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã�ѧϰ��ȫ����ջ���֪ʶ��
�ʴ�Ϊ��CN-+ClO-�TCNO-+Cl-��2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O��
��2��ʵ��ԭ��Ϊ��������ܢ����յĶ�����̼������ȷ����CN-�Ĵ�����������װ���в���N2��CO2��Cl2��H2O��������ˮ���ܱ���ʯ�����գ�Ӱ�������̼�����IJⶨ�����Խ������ܢ������Ӧ��ȥ������ˮ����Ũ������ˮ����ͭ����ȥ������ͬʱӦ��ֹ�����е�CO2��ˮ�����������ܢ����ʵ�飮�ʱ�װ���е��Լ���Ũ�����װ�õ�Ŀ���dz�ȥCl2������ܢ������������CO2������ܢ�������Ƿ�ֹ�����е�CO2��ˮ�����������ʵ�飮
�ʴ�Ϊ��Ũ���ᣬ��ȥCl2������CO2����ֹ�����е�CO2��ˮ�����������ʵ�飮
��3��װ���в�����CO2δ����ȫ���գ����²ⶨ�Ķ�����̼����ƫ�ͣ���õ�CN-�������İٷ�����ʵ��ֵ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�װ���в�����CO2δ����ȫ���գ�
��4������ܢ��м�ʯ������1.408gΪ������̼�����������ʵ���Ϊ
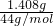 =0.032mol������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2��=0.032mol��ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol�����Ը�ʵ���в��CN-�������İٷ���Ϊ
=0.032mol������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2��=0.032mol��ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol�����Ը�ʵ���в��CN-�������İٷ���Ϊ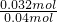 ��100%=80%��
��100%=80%���ʴ�Ϊ��80%
��������1�����������֪�����еķ�ӦΪNaClO��CN-����������CNO-�����еķ�ӦΪ����������CNO-��NaClO��Ӧ����N2��CO2��Cl2������������ԭ��Ӧ��ƽ��
��2��ʵ��ԭ��Ϊ��������ܢ����յĶ�����̼������ȷ����CN-�Ĵ�����������װ���в���N2��CO2��Cl2��H2O��������ˮ���ܱ���ʯ�����գ�Ӱ�������̼�����IJⶨ�����Խ������ܢ������Ӧ��ȥ������ˮ����Ũ������ˮ����ͭ����ȥ������ͬʱӦ��ֹ�����е�CO2��ˮ�����������ܢ����ʵ�飮
��3��װ���в�����CO2δ����ȫ���գ����²ⶨ�Ķ�����̼����ƫ�ͣ�
��4������ܢ��м�ʯ������1.408gΪ������̼������������̼Ԫ���غ��֪��������CN-�����ʵ���Ϊn��CN-��=n��CO2����ԭ��Һ��CN-�����ʵ���Ϊ0.2L��0.2mol/L=0.04mol���ݴ˼��㣮
����������ʵ��ԭ����ʵ��װ�����������ۡ����û�ѧ�����ѧ����ȣ��Ѷ��еȣ�����ʵ��ԭ���ǹؼ����Ƕ���ѧ֪ʶ���ۺ����ã�ѧϰ��ȫ����ջ���֪ʶ��

��ϰ��ϵ�д�
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
�����Ŀ
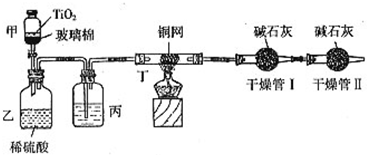 ��2010?����ģ�⣩���ݻ���Ҫ���ڴ��������Ʒ�ˮʱ���綾��CN-�ڴ���TiO2���������£�����NaClO��CN-������CNO-��CN-��CNO-��NԪ�ؾ�Ϊ-3�ۣ����������������¼�����NaClO��Ӧ����N2��CO2��Cl2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬�ⶨCN-�������İٷ��ʣ�
��2010?����ģ�⣩���ݻ���Ҫ���ڴ��������Ʒ�ˮʱ���綾��CN-�ڴ���TiO2���������£�����NaClO��CN-������CNO-��CN-��CNO-��NԪ�ؾ�Ϊ-3�ۣ����������������¼�����NaClO��Ӧ����N2��CO2��Cl2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬�ⶨCN-�������İٷ��ʣ�