��Ŀ����
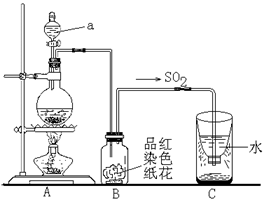
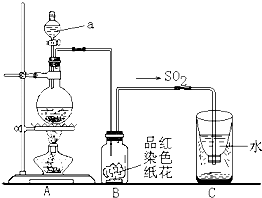
ʵ����ȡ��ϩʱ��Ӧ���Ҵ���Ũ������ټ��ȵ�170�棬��140��ʱ���������ѣ��¶ȹ���ʹ�����Ҵ���ŨH2SO4��Ӧ����SO2��CO2��ˮ������
��1���ֱ�д���Ҵ���ŨH2SO4��Ӧ���ɢ���ˮ����CH2=CH2���ڷ��Ӽ���ˮ����CH3CH2-O-CH2CH3����SO2��CO2��ˮ�����Ļ�ѧ����ʽ
�� ��
�� ��
�� ��
��2�����������Ǵ�����Ⱦ��֮һ����
���еĶ�����������ˮ�½��γ����ꡣ����ͼʵ��װ�ã���������γɽ���ģ�Ⲣ��֤��������IJ������ʣ���ش�
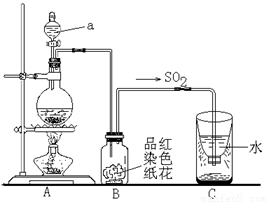
��ָ��ͼ������a�����ƣ� ��
��Bװ���е���ɫֽ������ɫ����ȥ��˵��SO2�� �ԡ�
�۷�Ӧ������ȡ��Cװ��������Һ�壬�μ���ɫʯ����Һ��� ɫ��
��ʵ����Ϻ�Ϊ��ʹ����Ķ�����������գ�Cװ��Ӧ��θĽ���
��������˵���� ��
�� ��CH3CH2OH  CH2=CH2
+ H2O
CH2=CH2
+ H2O
�� 2CH3CH2OH
 CH3CH2-O-CH2CH3
+ H2O
CH3CH2-O-CH2CH3
+ H2O
�� CH3CH2OH
+ H2SO4 (Ũ) SO2 ��+CO2
��+ H2O
SO2 ��+CO2
��+ H2O
�� ��Һ©����Ư�� ��ɫ ��C�м����������������Һ������
����������1�������Ҵ��Ļ�ѧ�����Լ�����ʽ����д���Ҵ��к����ǻ����Է�����ȥ��Ӧ������ϩ��Ҳ���Է������Ӽ����ˮ��Ӧ�������ѡ���ΪŨ���ỹ����ǿ�����ԣ��ڼ��ȵ��������Ҵ����Ա�Ũ������������CO2����Ũ������ԭ����SO2��
��2�����������Ľṹ�ص���ж�a�Ƿ�Һ©����SO2����Ư���ԣ���ʹƷ����Һ��ɫ��ͬʱSO2����һ���������������ˮ���������������ԣ�������ɫʯ����Һ�Ժ�ɫ����ΪSO2��һ�ִ�����ȾΪ����Ҫ����β��������������������������Һ�����ն����SO2��

 ʵ����ȡ��ϩʱ��Ӧ���Ҵ���Ũ������ټ��ȵ�170�棬��140��ʱ���������ѣ��¶ȹ���ʹ�����Ҵ���ŨH2SO4��Ӧ����SO2��CO2��ˮ������
ʵ����ȡ��ϩʱ��Ӧ���Ҵ���Ũ������ټ��ȵ�170�棬��140��ʱ���������ѣ��¶ȹ���ʹ�����Ҵ���ŨH2SO4��Ӧ����SO2��CO2��ˮ������