��Ŀ����
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ������
��Ŀǰ��ҵ�Ͽ�����CO2������CH3CH2OH����ӦΪ��2CO2(g)+6H2(g) CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���

���ݱ������ݷ�����
��1���÷�Ӧ������Ӧ��_________��ѡ����ȡ����ȡ�����Ӧ��
��2�������̼��n(H2)/n(CO2)���������Ҵ� _______��ѡ���������������������Ӱ�족����
��3�������Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2�����д�ʩ����ʹc(CH3CH2OH)�������_______������ĸ����ͬ����
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������ D���ٳ���1molCO2��3molH2
��ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)
��4����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ___________��
��5��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����___________��
A��c(CO)=c(H2) B��v��(CO)=v��(H2O)
C�������е�ѹǿ���� D������2molCO��ͬʱ����1molCH3CH2OH
��6������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���______________________����CO2��ȡCH3CH2OH���ŵ���____________����д��һ�㼴�ɣ�
��1������ (1��)
��2������ (2��)
��3��C��D (2��)
��4��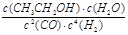 (2��)
(2��)
��5��C (2��)
��6��ʹԭ���нϴ��ת���� CO2ԭ���õ� (2��) �����������𰸾��ɸ��֣�
��������

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д���̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ��������ͼ���ɶ�����̼�ϳ��Ҵ��ļ������̣�
���ճ���ʢ�б���̼�����Һ���Ѻ��ж�����̼�Ŀ����������ճ��У����ճ��з�ӦҺ����ֽ�غ���ֽ����ͨ�����ˮ�������Ѷ�����̼����Һ����ȡ�������ںϳ����к���������ѧ��Ӧʹ֮��Ϊ������ȼ���Ҵ����ش��������⣺
��1��д�����ճ��з�Ӧ�����ӷ���ʽ ��
��2���ӷֽ����ѭ��ʹ�õ������� ��
��3����ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g)CH3CH2OH(g)+H2O(g)
д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ K= ��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH ��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���ʹԭ���нϴ��ת���ʣ���CO2��ȡCH3CH2OH���ŵ��� ����д��һ�㼴�ɣ�
|
| 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2.0 | 60 | 43 | 28 | 15 |
| 3.0 | 83 | 62 | 37 | 22 |
��5����һ��ѹǿ�£������CO2��ȡCH3CH2OH��ʵ���������±���
���ݱ������ݷ�����
���¶����ߣ��÷�Ӧ��ƽ�ⳣ��Kֵ ��ѡ���������С�����䡱����
�������̼n(H2)/n(CO2)��,�������Ҵ� ��ѡ���������������������Ӱ�족����

 ��̼��������ֱ�Ӻϳɼ״����Ҵ�ȼ���ѽ��빤ҵ�������磺
��̼��������ֱ�Ӻϳɼ״����Ҵ�ȼ���ѽ��빤ҵ�������磺

 CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)